Biotechnology
Innovent Dosed First Participant in Phase 3 Clinical Study (Neoshot) of IBI310 (Anti-CTLA-4 Monoclonal Antibody) in Combination with Sintilimab for MSI-H/dMMR Colon Cancer Neoadjuvant Therapy
ROCKVILLE, Md. and SUZHOU, China, March 27, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology a...
Bridge Biotherapeutics Announces a Research Collaboration with the University of Colorado School of Medicine to Explore Potential of BBT-877 for Immuno-Oncology
SEONGNAM, South Korea and AURORA, Colo., March 26, 2024 /PRNewswire/ -- Bridge
Biotherapeutics (KQ288330)
World First Use of Lightpoint's SENSEI® Drop-In Gamma Probe in Bladder Cancer Surgery Performed in Spain
LONDON, March 27, 2024 /PRNewswire/ -- Lightpoint Surgical Limited (Lightpoint), an affiliate of Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces a world-first bladder cancer sentinel lymph node (SLN) procedure with SENSEI®, Telix's miniature robotic-assisted gamma pro...
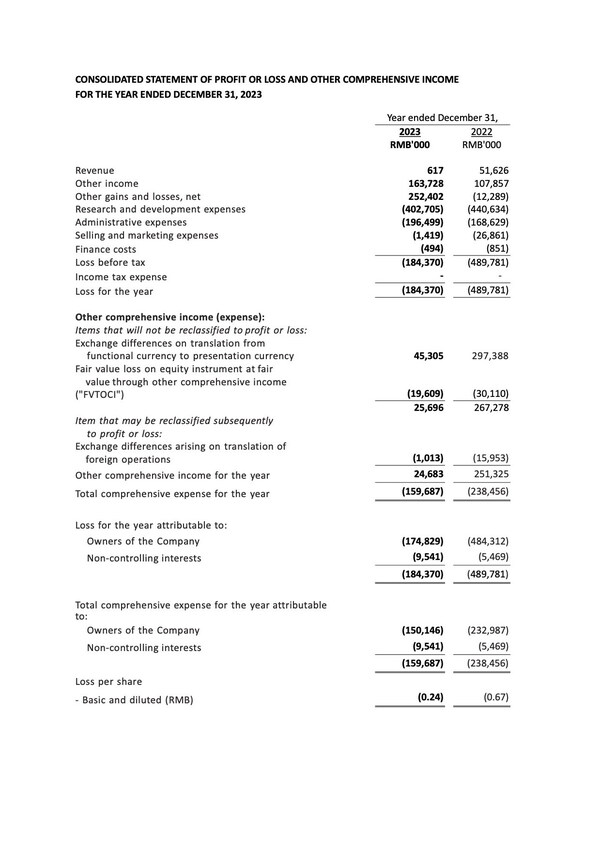
WuXi Biologics Reports Solid 2023 Annual Results
Revenue Increased by 11.6% Y-o-Y to RMB17,034.3 Million Gross Profit Rose by 1.5% to RMB6,827.9 Million Non-COVID Revenue Achieved 37.7% Y-o-Y Growth, Strong Momentum Sustained Non-COVID Late-Phase and Commercial Manufacturing Revenue Grew by 101.7% Y-o-Y "R" in CRDMO Thrived with Extended Part...
ReportLinker Reveals Key Insights About Players and Coverage in Latest Combination Vaccine Market Analysis
LYON, France, March 26, 2024 /PRNewswire/ -- Reportlinker announces the release
of the report "A White Paper To Understand The Market Structure Of Pediatric
Pertussis Hexavalent and Combination Vaccines
Lunit SCOPE IO Reveals Promising Results in Neoadjuvant Immunotherapy Study for Head and Neck Cancer Patients
- New research published in Clinical Cancer Research shows significant improvement in distant recurrence-free survival with combined durvalumab and tremelimumab treatment, supported by Lunit SCOPE IO's analysis of tumor microenvironment SEOUL, South Korea, March 26, 2024 /PRNewswire/ -- Lunit (K...
TiumBio Submits CTA for Phase 1b Clinical Trial of TU7710, a Long-acting Recombinant Activated Factor VII, in Hemophilia A or B Patients
* TU7710 has the potential to be a highly effective and long-acting treatment for bleeding episodes as well as for preventing bleeding during surgery or invasive procedures in hemophilia patients with inhibitors * Interim results from an ongoing Phase 1a study in healthy male volunteers will ...
Alphamab Oncology Announces the First Patient Dosed in Australia in the Clinical Study of Subcutaneous Formulation JSKN033
SUZHOU, China, March 26, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the first patient has been dosed inAustralia in the clinical study (JSKN033-101) of JSKN033, a subcutaneous formulation with HER2 bispecific antibody-conjugated drug and PD-L1 monoclonal antibody,...
Scivita Medical Expands Collaboration with Boston Scientific
SUZHOU, China, March 26, 2024 /PRNewswire/ -- Recently, Scivita Medical
Technology Co., Ltd. ("Scivita Medical") and Boston
Scientific Corporation (NYSE: BSX), a leading global medical technology
company, joined hands again to sign an expanded strategic cooperation
arrangement.
BioDuro-Sundia's Partner, DigmBio Announced IND Clearance from Korea MFDS for its Selective PARP1 Inhibitor DM5167
SHANGHAI, March 26, 2024 /PRNewswire/ -- BioDuro-Sundia's partner, DigmBio, a South Korean biotechnology company, announced its selective PARP1 inhibitor for the treatment of triple-negative breast cancer has been approved by Korea Food and Drug Administration (MFDS) for Investigational New Drug ...
Full-Life Technologies Achieves Regulatory Milestone with Nuclear Permit, Advancing Radioisotope Facility Construction
GEMBLOUX, Belgium, March 25, 2024 /PRNewswire/ -- Full-Life Technologies ("Full-Life"), today announced the recent achievement of its subsidiary "Full-Life Technology Europe" has obtained a Nuclear Permit for a class IIA facility from the Belgium Federal Agency for Nuclear Control ("FANC"), auth...
GC Genome to Present New Data at AACR 2024
Offering Hope for Enhanced Early Detection and Patient Care in the Battle Against Cancer YONGIN, South Korea, March 25, 2024 /PRNewswire/ -- GC Genome Corporation, a leading diagnostics company, today announced that the company will present two poster presentations at the American Association fo...
Medipledge, a premium post-laser skincare brand, just launched in Australia
SYDNEY, March 25, 2024 /PRNewswire/ -- On March 24th, 2024, Medipledge, a premium skincare brand developed by experts in beauty and dermatology, successfully launched inAustralia. The launch event, held in Australia, featured high-tech elements and included high-end aesthetic therapists, lead...
Advancing Healthcare Innovation: Juniper Biologics Recognized as the Best Novel Therapy R&D and Commercialization Company 2024 by APAC Insider
SINGAPORE, March 25, 2024 /PRNewswire/ -- Juniper Biologics
Veeva Vault EDC Surpasses 1,000 Study Start Milestone
Eight of the top 20 biopharmas standardize on Vault EDC to build a modern
clinical data foundation
SINGAPORE, March 25, 2024 /PRNewswire/ -- Veeva Systems
Brii Biosciences Provides Corporate Update and Reports Full-Year 2023 Financial Results
Transitioning HBV cure programs into multiple late-stage combination studies with interim results throughout 2024 and 2025 informing Company's registrational strategy Integrating R&D, manufacturing and commercial upsides by acquiring full intellectual property rights of BRII-179 and expanding it...
The Results of Phase II Clinical Study of KN046 Plus Chemotherapy as First-line Treatment for Metastatic NSCLC were Published in Cell Reports Medicine
SUZHOU, China, March 22, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the phase II clinical research results of anti- PD-L1/CTLA-4 bispecific antibody KN046 plus chemotherapy as first-line treatment for metastatic non-small cell lung cancer (NSCLC) were published o...
Antengene Announces Full Year 2023 Financial Results, Highlights Clinical Progress Across First-in-Class, Best-in-Class Pipeline
* Promising clinical activities and efficacies during dose escalations for four lead global rights programs targeting CD24, Claudin 18.2, CD73, and PD-L1/4-1BB * Positive, differentiated cervical cancer data advancing mTORC1/2 inhibitor on registrational track for APAC markets * RMB1.188 bi...
NeuExcell Therapeutics Announces Successful Dosing of First Patient by NeuroD1 Gene Therapy
SUZHOU, China, March 22, 2024 /PRNewswire/ -- NeuExcell Therapeutics, a leading biotechnology company focused onin vivo neural regenerative therapies, announces the successful dosing of the first patient with the first-in-class NeuroD1 gene therapy product NXL-004. This milestone represents si...
100% CBR! FDA Granted ODD to Biostar Pharma's Utidelone Capsule (UTD2) for the Treatment of Gastric Cancer
SAN FRANCISCO, March 21, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the U.S. subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the development and commercialization of innovative oncology drugs, announced today that their ...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 292 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 290 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00