Biotechnology
Hayashibara Brings Back "Trehalose Symposium" to Reveal Its Game-changing Versatilities in Human Biochemistry
OKAYAMA, Japan, Oct. 4, 2022 /PRNewswire/ -- Hayashibara Co., Ltd., a member of Nagase Group and headquartered in Okayama City, westernJapan, hosted the 24th Trehalose Symposium, with the support of the Japanese Society of Applied Glycoscience, onSeptember 8, 2022. Photo: https://kyodonewsprwir...
Kira Pharmaceuticals Receives FDA Clearance of IND Application for Phase 2 Evaluation of KP104 in Systemic Lupus Erythematosus Associated Thrombotic Microangiopathy (SLE-TMA)
Phase 1 supporting data indicated an encouraging profile and confirmed the dual mechanism of action achieved through targeting both the alternative and terminal complement pathways CAMBRIDGE, Mass., Oct. 3, 2022 /PRNewswire/ -- Kira Pharmaceuticals, a global biotechnology company pioneering tran...
DEBIOPHARM REINFORCES THE BOND BETWEEN SWISS AND JAPANESE CANCER RESEARCH WITH THE 2022 JCA MAUVERNAY AWARD CEREMONY
Debiopharm awards the winning scientists for the JCA (Japanese Cancer
Association) Award for innovative, disruptive, and translational oncology
research
LAUSANNE, Switzerland, Oct. 3, 2022 /PRNewswire/ -- Debiopharm (
www.debiopharm.com
YS BIOPHARMA TO MERGE WITH NASDAQ-LISTED SUMMIT HEALTHCARE ACQUISITION CORP.
* Transaction values YS Biopharma at pre-money equity value of $834 million * Certain investors ("Forward Purchase Investors") are expected to invest $30 million in a private placement concurrently with the closing of the business combination transaction pursuant to certain forward purchase ag...
Samsung Biologics introduces new development platforms S-DUAL™ and DEVELOPICK™ at BPI Boston 2022
* Two distinctive platforms will streamline the antibody development process. * With an advanced design and high binding affinity, S-DUAL™ ensures optimized manufacturability of bispecific antibodies. * DEVELOPICK™ provides early insight and selection guidance to save time and cost to maxi...
NeuShen Therapeutics Closes Pre-A Financing with ~$20M
* Closure of pre-A financing round led by LAMPAM Capital * Dual internal discovery platforms of small molecule and AAV-based gene therapy targeting neurological and psychiatric disorders SHANGHAI, Sept. 29, 2022 /PRNewswire/ -- NeuShen Therapeutics, Inc., a biotechnology company focusing on d...
Kintor Pharma Announces Publication of Phase Ib Data from Pruxelutamide for AR+ mBC in EJC
SUZHOU, China, Sept. 29, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that the results from a phase Ib study of pruxelutamide (used to be ...
WuXi Biologics Topped Off the Steel on its Commercial Biomanufacturing Facility in Worcester, Massachusetts
WORCESTER, Mass., Sept. 29, 2022 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global Contract Research, Development and Manufacturing Organization (CRDMO), held a topping-off ceremony, in partnership with the Worcester Business Development Corporation (WBDC), to mark the completion of...
Green Mountain Biotech and Kamedis Announce First Patent in China for Unique Herb-Based Acne and Skin Relief Solution
Clinically-tested solution, based on a proprietary blend of six herbs used in
Traditional Chinese Medicine, helps relieve symptoms of acne and other skin
inflammation conditions.
TEL AVIV, Israel, Sept. 29, 2022 /PRNewswire/ -- Green Mountain Biotech
GC Labs becomes the first Korean laboratory to obtain certification of CDC standardization programs (VDSCP & HoSt)
YONGIN, South Korea and AUSTIN, Texas, Sept. 28, 2022 /PRNewswire/ -- GC Labs, a leadingSouth Korea clinical laboratory, today announced that it has recently obtained certification of standardization programs from the U.S. Centers for Disease Control and Prevention (CDC) for vitamin D and hormone...
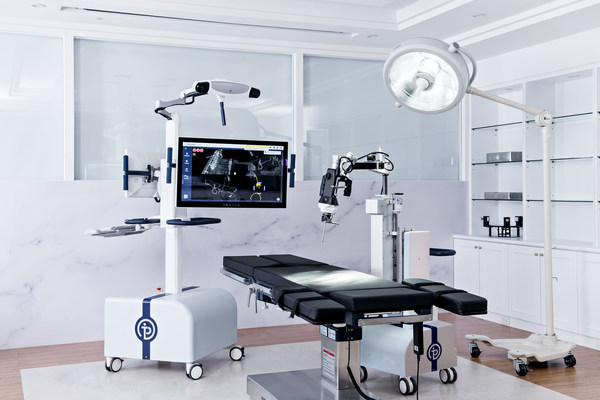
First-Ever Spinal Surgical Hand-held Robot by Point Robotics MedTech to Make Its Worldwide Debut in the United States
TAIPEI, Sept. 28, 2022 /PRNewswire/ -- Point Robotics MedTech Inc. (Point Robotics), a rising star in the field of orthopedic surgery, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its minimally invasive surgical robot, POINT™ Kinguide Robotic-Assisted Surgical Sy...
ASTRUM-005: The first immunotherapy clinical study of SCLC published in JAMA, one of the top medical journals in the world
* ASTRUM-005 is the first study to demonstrate that PD-1 inhibitors plus chemotherapy can improve survival for patients with ES-SCLC. * In ASTRUM-005, serplulimab combined with chemotherapy achieved by far the longest OS in first-line immunotherapy for ES-SCLC. Compared with chemotherapy, the...
ICHNOS SCIENCES ANNOUNCES INITIATION OF FIRST-IN-HUMAN STUDY FOR ISB 1442
First patient dosed with novel biparatopic 2+1 BEAT® bispecific antibody targeting CD38 and CD47 NEW YORK, Sept. 28, 2022 /PRNewswire/ -- Ichnos Sciences Inc., a global clinical-stage biotechnology company developing innovative multispecific immune cell engager antibodies in oncology, today anno...
Advanced Instruments Introduces Solentim VIPS™ PRO, A High Efficiency Single Cell Seeder to Optimize Cell Line Development Workflows
VIPS PRO delivers powerful and efficient seeding technology and definitive proof of monoclonality for workflow productivity and confidence. NORWOOD, Mass., Sept. 28, 2022 /PRNewswire/ -- Advanced Instruments today announced the launch ofSolentim VIPS PRO, a high efficiency single cell seeder, at...
Samsung Biologics Earns Gold Rating from EcoVadis for Its Sustainability Efforts
* Rising from last year's ranking, the company's united effort and dedication to integrating sustainable practices in all areas of its business drew recognition from EcoVadis, which provided high marks in responsible procurement and environmental management. * More than 100,000 companies from...
Portfolio Highlights: Clinical and Financial Updates of ABM, F5, AceLink, HAYA, Regenacy, and Arthrosi
SHANGHAI, Sept. 28, 2022 /PRNewswire/ -- As the investment division of Viva Biotech, Viva BioInnovator is committed to being a collaborative platform for Innovative Biotech companies from around the world. Over the past 2 months, our portfolio companies progressed greatly. ABM Therapeutics Annou...
The 6th China (Shenzhen) Innovation & Entrepreneurship International Competition Eindhoven, Netherlands Division is Open for Registration
DIGITAL ACCESS TO THE WORLD CREATES A BETTER FUTURE EINDHOVEN, Netherlands, Sept. 28, 2022 /PRNewswire/ -- China (Shenzhen)Innovation and Entrepreneurship Competition, known as the "Olympics" of innovation and entrepreneurship circle, is here again! The sixth International competition was offici...
Biosyngen Gives Cell Therapy GMP Capabilities in Singapore A Boost
SINGAPORE, Sept. 28, 2022 /PRNewswire/ -- On September 22nd, Biosyngen signed to set up a Cell Therapy GMP Facility which will serve as a base to develop the company's immunotherapy assets to address unmet needs in cancer treatment. Located in Solaris @ Tai Seng, this 1,300 square metres faci...
Vernalis Research, a fully owned subsidiary of HitGen Inc., and Contera Pharma A/S announce a strategic collaboration in the field of RNA-targeting small molecules in neurology
CAMBRIDGE, England and COPENHAGEN, Denmark, Sept. 28, 2022 /PRNewswire/ -- Vernalis Research ("Vernalis"), a fully owned subsidiary of HitGen Inc., and Contera Pharma A/S ("Contera") are pleased to announce a strategic drug discovery collaboration on an undisclosed target. Under the terms of the...
Pharming Announces US FDA Acceptance for Priority Review of its New Drug Application for Leniolisib
The FDA has assigned a PDUFA goal date of March 29, 2023 for the NDA submission based on randomized-controlled and long-term extension data for leniolisib as a treatment for APDS, a rare primary immunodeficiency LEIDEN, The Netherlands, Sept. 28, 2022 /PRNewswire/ -- Pharming Group N.V. ("Pharmi...
Week's Top Stories
Most Reposted
Protiviti Transforms Cyber Risk Consulting with CYFIRMA's Advanced Intelligence-Led Cybersecurity
[Picked up by 324 media titles]
2024-04-15 09:00Sephora appoints Xia Ding as Managing Director of Sephora Greater China
[Picked up by 309 media titles]
2024-04-15 17:33With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 281 media titles]
2024-04-18 21:26Animated Life in Yunnan
[Picked up by 278 media titles]
2024-04-18 14:00Allianz Partners Introduces Instant Online Chat for HCF Travel Insurance Customers
[Picked up by 276 media titles]
2024-04-15 08:00