Medical/Pharmaceuticals
China Pharma Announced the Submission of a Plan of Compliance to NYSE American
HAIKOU, China, July 18, 2022 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma," the "Company" or "We"), a specialty pharmaceutical company, today announced that onJuly 15, 2022, the Company submitted a plan of compliance to NYSE American LLC ("NYSE American" or the...
Samsung Biologics to purchase land for its second Bio Campus
* Acquires additional land, sized 357,366㎡ valued at KRW 426 billion, for Bio Campus 2 * Plans to build Multi-Modal Plant, Open Innovation, and expanded manufacturing capacities INCHEON, South Korea, July 18, 2022 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), the world's leading contrac...
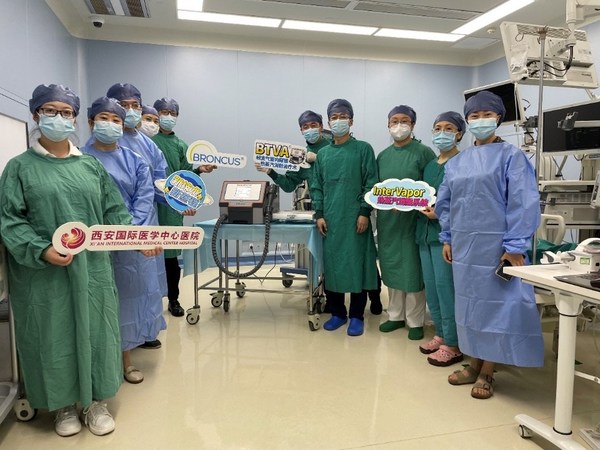
BRONCUS COMPLETED SURGERIES ON THE FIRST GROUP OF PATIENTS USING ITS InterVapor®, THE THERMAL VAPOR TREATMENT SYSTEM, AFTER APPROVED FOR MARKETING IN CHINA
HANGZHOU, China, July 18, 2022 /PRNewswire/ -- Broncus Medical (02216.HK) has completed surgeries on the first group of patients using its InterVapor® (including InterVapor Generator and InterVapor Catheter, the "InterVapor®"), after it was approved for marketing inChina. This marked the official ...
Everest Medicines Announces European Commission Grants Approval of Kinpeygo® for Adults with Primary IgA Nephropathy to our Partner Calliditas Therapeutics
Kinpeygo® (developed under the name NEFECON) is the first and only EMA- approved treatment for IgAN Everest Medicines has exclusive rights to develop and commercialize NEFECON in Greater China, Singapore and South Korea SHANGHAI, July 17, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Ev...
Everest Medicines Announces European Commission Grants Approval of Kinpeygo® for Adults with Primary IgA Nephropathy to our Partner Calliditas Therapeutics
Kinpeygo® (developed under the name NEFECON) is the first and only EMA- approved treatment for IgAN Everest Medicines has exclusive rights to develop and commercialize NEFECON in Greater China, Singapore and South Korea SHANGHAI, July 18, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Ev...
Amaris B. Clinic Is Seeing More Patients for Liposuction Due To Inefficacy of Non-surgical Fat Reduction Treatments
Dr Ivan Puah, Medical Director at Amaris B. Clinic, has been performing modern liposuction and corrective surgeries for patients who have experienced less than satisfactory results from non-surgical treatments and those who suffer from PAH, a rare side effect of cryolipolysis. SINGAPORE, Ju...
China CDE Grants Breakthrough Therapy Designation (BTD) to Neurophth's NR082 in LHON
WUHAN, China and SAN DIEGO, July 15, 2022 /PRNewswire/ -- Neurophth Therapeutics, Inc., ("Neurophth") today announced thatthe Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has granted a Breakthrough Therapy Designation (BTD) to the Company's leading gen...
Pulnovo Medical Concludes Successfully First Pre-Sub Meeting with FDA for PADN Global Clinical Trial
SHANGHAI, July 15, 2022 /PRNewswire/ -- Pulnovo Medical Limited, a globally recognized device pioneer in the treatment for cardiopulmonary diseases, today announced that it has successfully concluded the first Pre-Sub meeting with the U.S. Food and Drug Administration ("FDA") for its Pulmonary Ar...
CStone Announces the NDA Approval of GAVRETO® (pralsetinib) for the Treatment of RET Fusion-Positive treatment-naïve (first-line) and pretreated Non-Small Cell Lung Cancer (NSCLC) in Hong Kong, China
* GAVRETO is the first highly selective rearranged during transfection (RET) inhibitor approved inHong Kong, China for the treatment of RET fusion-positive metastatic non-small cell lung cancer * GAVRETO is CStone's second therapy approved in Hong Kong, China * GAVRETO is CStone's ninth NDA ...
Zymo Research's DNA/RNA Shield™ Inactivates Monkeypox for the Collection and Transportation of Samples
IRVINE, Calif., July 15, 2022 /PRNewswire/ -- Zymo Research Corp., in collaboration with the Institute of Virology, University Medical Center Freiburg,Germany, demonstrated data showing their DNA/RNA Shield™ Inactivating Transport Medium (ITM) completely inactivated recent Monkeypox virus isolate...
Lunit to be Listed on KOSDAQ next week
* Initial Public Offering of 1,215,800 shares of its common stock at KRW 30,000 * To be listed on the KOSDAQ market on July 21, 2022 SEOUL, South Korea, July 14, 2022 /PRNewswire/ -- Lunit, a global provider of AI-based cancer solutions, announced that it priced its initial public offering (I...
Urgent support needed following the pandemic's effect on cancer screening services
Dry July call to arms will improve support for cancer patients SYDNEY, July 14, 2022 /PRNewswire/ -- Dry July Foundation is urging Australians to dig even deeper this Dry July to help people affected by cancer. So many Australians could not access vital lifesaving cancer screenings and treatment...
Tianlong launched a campaign to care for SMA children
XI'AN China, July 14, 2022 /PRNewswire/ -- Tianlong has launched a campaign together with the Spinal muscular atrophy (SMA) care center inChina (a non-profit organization for SMA), and The Second Affiliated Hospital of Xi'an Jiaotong University to care for children with SMA inMay 2022. Spinal mu...
Oncoshot Receives Funding To Expand Cancer Data Sharing Capabilities For Hospitals and Industry
SINGAPORE, July 14, 2022 /PRNewswire/ -- Oncoshot today announced a pre-series A funding round led by MassMutual Ventures (MMV). Other investors for this stage come from a suite of healthtech veterans that include Milltrust International and health industry angel investors. This funding wi...
Nanoform and Pharmanovia to breathe new life into iconic medicines
HELSINKI, July 14, 2022 /PRNewswire/ -- Nanoform Finland Plc., an innovative nanoparticle medicine-enabling company, today announced that it has partnered with Pharmanovia, a fast-growing specialty pharma business with a portfolio of over 20 branded drugs in 140 markets. The new strategic partne...
GenScript ProBio and DAAN Bio Therapeutics, Sign a Strategic Cooperation MOU for the Discovery and Development of Novel Drugs
SEOUL, South Korea, July 14, 2022 /PRNewswire/ -- On the July 14, 2022, GenScript ProBio (Brian Ho-sung Min, CEO), a global CDMO, and DAAN Bio Therapeutics (Byoung Chul Cho, Co-Founder & CEO), an innovative new drug developer such as antibody treatments and cell therapy drugs, announced that the...
Triastek announces research collaboration with Lilly to explore the application of 3D printing technology in oral delivery of drugs
NANJING, China, July 14, 2022 /PRNewswire/ -- On July 13, 2022, Triastek, Inc. ("Triastek") announced a collaboration with Eli Lilly and Company ("Lilly"), a leading global pharmaceutical company, to leverage the advantages of 3D printing technology to enable precisely targeted and programmed rel...
U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted for Individuals Aged 18 and Over
* Novavax' vaccine is the first protein-based COVID-19 vaccine authorized in the U.S. * Immunizations with the Novavax COVID-19 Vaccine, Adjuvanted as a primary series will begin upon product release and once a policy recommendation from the CDC is received GAITHERSBURG, Md., July 14, 2022 /P...
miR Scientific is proud to announce the commercial availability of the miR Sentinel™ Prostate Cancer Test
NEW YORK, July 14, 2022 /PRNewswire/ -- miR Scientific, LLC announces the miR Sentinel™ Prostate Cancer Test is now commercially available in the United States, Puerto Rico and select international markets. miR Sentinel™ is a novel, urine-based, molecular test that analyzes small non-coding RNA ...
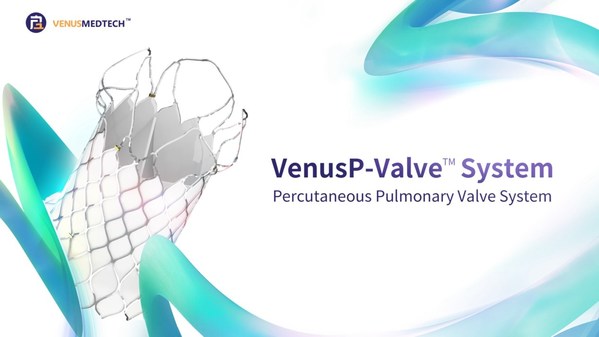
Filling gap in market: VenusP-Valve™ approved by China's NMPA
HANGZHOU, China, July 13, 2022 /PRNewswire/ -- On July 11, 2022, VenusP-ValveTM , Venus Medtech's in-house developed innovative transcatheter pulmonic valve replacement (TPVR) system, was approved byChina's National Medical Products Administration (NMPA) for the treatment of severe pulmonary regur...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 292 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 290 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00