Medical/Pharmaceuticals
United Imaging Shows Up Big, Differently
Focusing squarely on its Equal Healthcare for All™ mission, the company had its flagship digital PET/CT driven across the border on a trailer to SNMMI 2022. HOUSTON, June 14, 2022 /PRNewswire/ -- United Imaging, a global leader in manufacturing advanced medical imaging and radiotherapy equipment,...
Zhimeng Biopharma will report positive phase 1b trial results on its HBV capsid inhibitor ZM-H1505R in the upcoming International Liver Congress
SHANGHAI, June 14, 2022 /PRNewswire/ -- Shanghai Zhimeng Biopharma announces that it will report the positive results obtained from a phase1b study on its HBV capsid inhibitor ZM-H1505R in the International Liver Congress inJune 22-26, 2022 (the EASL meeting). The recently completed phase 1b stu...
Telix and Invicro Advance AI Partnership
MELBOURNE, Australia and BOSTON, June 14, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that it has advanced a partnership with Invicro LLC (Invicro), a global, industry-leading imaging CRO, and part of REALM IDx, Inc., to develop an artificial...
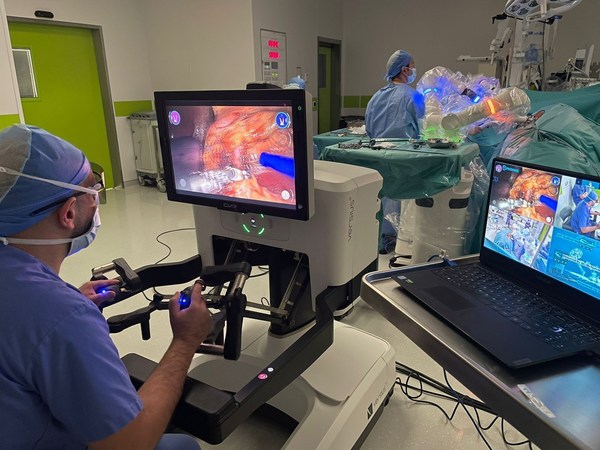
PROXIMIE RAISES $80 MILLION IN SERIES C FUNDING TO ACCELERATE PRODUCT EXPANSION OF FULL-SERVICE CONNECTED SURGICAL PLATFORM
Funding round led by Advent Life Sciences. New investors include Emerson Collective, SoftBank Vision Fund 2, British Patient Capital, Mubadala and the Minderoo Foundation. Proceeds to accelerate development and scale of Proximie's Operating System for the Operating Room - a centralized platform ...
Cellenkos Receives FDA Clearance of Investigational New Drug (IND) Application for CK0804 as Add on Therapy to Ruxolitinib for the Treatment of Myelofibrosis
-- CK0804 represents the company's fourth program to receive IND Clearance -- CK0804 is a partnered program with Incyte HOUSTON, June 14, 2022 /PRNewswire/ -- Cellenkos, Inc., a privately held, clinical stage biotech company that focuses on developing transformative T regulatory cell therapies ...
Telix and Invicro Advance AI Partnership
MELBOURNE, Australia and BOSTON, June 14, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces that it has advanced a partnership with Invicro LLC (Invicro), a global, industry-leading imaging CRO, and part of REALM IDx, Inc., to develop an artificial...
Kintor Pharma and Etana's Collaboration on Pruxelutamide's COVID-19 Project Awarded with Belt and Road Innovation Project and Fund Support from the Science and Technology Department of Jiangsu Province
SUZHOU, China, June 14, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that its "clinical trial cooperative research and development and ov...
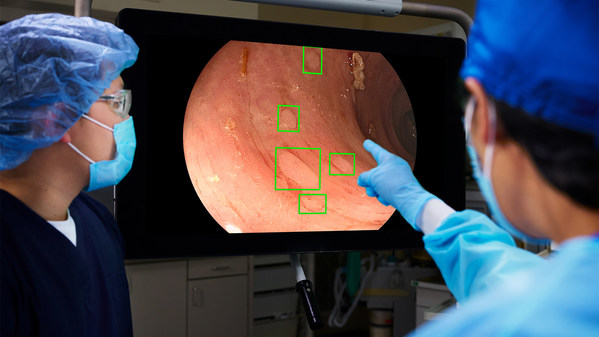
Farrer Park Hospital Taps on AI to Improve Colorectal Cancer Screening Results
Enhanced detection allows for higher polyp detection rates and more effective screening for colorectal cancer SINGAPORE, June 14, 2022 /PRNewswire/ -- Farrer Park Hospital ("FPH" or "the Hospital") today announced that it has introduced artificial intelligence ("AI") assisted colorectal screenin...
Alphamab Oncology Announced First Patient Dosed in Phase I Trial of PD-L1/OX40 Bispecific Antibody KN052
SUZHOU, China, June 14, 2022 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the first patient was dosed in a Phase I clinical study (KN052-CHN-001) of KN052, its proprietary PD-L1/OX40 bispecific antibody, in patients with advanced solid tumors inChina. With the developme...
Innovent and Etana Jointly Announce the Approval of Bevagen® (Bevacizumab Biosimilar) by the Indonesian Food and Drugs Authority (BPOM)
SAN FRANCISCO and SUZHOU, China, June 14, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and...
Innovent Announces the NMPA Acceptance of the New Drug Application for Tafolecimab Injection (anti-PCSK-9 antibody)
SAN FRANCISCO and SUZHOU, China, June 14, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and...
Treadwell Announces Initiation of Patient Dosing in TWT-203, a Phase 1b/2 study of TTK Inhibitor, CFI-402257, in Patients with ER+/Her2- Breast cancer
NEW YORK and HONG KONG, June 14, 2022 /PRNewswire/ -- Treadwell Therapeutics, a clinical-stage biotechnology company developing novel medicines for unmet needs in cancer, today announced the initiation of patient dosing in TWT-203, its Phase1b/2 study to evaluate CFI-402257, an oral, best-in-clas...
Good News from Taiwan Participants in BIO 2022 - Pharmasaga and PrecisemAb Make It to the Start-Up Stadium Finalists Defeating Hundred Teams
TAIPEI, June 13, 2022 /PRNewswire/ -- As the post-pandemic era approaches, the biomedical industry has become a strategic domain of focal emphasis inTaiwan and would be the critical impetus for the next wave of growth. In order to step up international connection and strive for business opportuni...
Dompé Announces Results of Phase 2 Study Evaluating the Efficacy and Safety of Reparixin in Patients with Severe COVID-19 Pneumonia
* In hospitalized patients with COVID-19 Pneumonia, the rates of clinical events among those treated with Reparixin (n=36) were statistically significantly lower than standard of care (n=19). * The study is the first evaluation of the efficacy and safety of Reparixin an IL-8 inhibitor in pati...
META Pharmaceuticals, a pioneer biotech in immune-metabolism, raised 15M USD to accelerate its pipeline development
SHENZHEN, China, June 13, 2022 /PRNewswire/ -- Today, META Pharmaceuticals, China's first immunometabolism-based small-molecule drug discovery company, announced two consecutive Seed and Pre-A funding rounds totaling15 million USD. Investors include Forcefield Ventures, XtalPi Inc., IMO Ventures,...
Jubilant Radiopharma Joins Illuccix® Pharmacy Partner Network
MELBOURNE, Australia and INDIANAPOLIS, June 13, 2022 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) is pleased to announce that its prostate cancer imaging agent, Illuccix® (kit for preparation of gallium Ga 68 gozetotide), is now available at selected pharmacies in ...
Novavax COVID-19 Vaccine Nuvaxovid™ Provisionally Registered in Australia as a Booster in Individuals Aged 18 and Over
* In Australia, Nuvaxovid™ is the first protein-based COVID-19 vaccine registered for use as a booster regardless of previous vaccine history GAITHERSBURG, Md., June 13, 2022 /PRNewswire/ -- Novavax, Inc. (NASDAQ: NVAX), a biotechnology company dedicated to developing and commercializing next-...
POSITIVE DATA FOR PAXALISIB IN TWO CHILDHOOD BRAIN CANCERS PRESENTED AT 20TH ISPNO ANNUAL MEETING
SYDNEY, June 13, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce the presentation of new data regarding the activity of paxalisib in two forms of childhood brain cancer with poor prognosis and limited t...
Curocell announced impressive CR rate in Phase 1 study with anbal-cel, the next generation CD19 CAR-T integrated OVIS™ platform
- 82% ORR (9 of 11 patients) and 82% (9 of 11 patients) CR rate documented after anbal-cel treatment to the patients in relapsed/refractory large B-cell lymphoma - 2 out of 3 patients dosed with 2x105 cells/kg of anbal-cel lasting the complete response for more than 12 months - Well tolerat...
Zhongchao Inc. Announces its MDMOOC Platform Launched Disease Prevention and Distribution Quality Management Course
SHANGHAI, June 13, 2022 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), an internet technology company offering healthcare professionals the online healthcare information, professional training and educational services platform and patient management service, today ...
Week's Top Stories
Most Reposted
Protiviti Transforms Cyber Risk Consulting with CYFIRMA's Advanced Intelligence-Led Cybersecurity
[Picked up by 324 media titles]
2024-04-15 09:00Sephora appoints Xia Ding as Managing Director of Sephora Greater China
[Picked up by 309 media titles]
2024-04-15 17:33AI-DOL: Revolutionising the Future of Entertainment with AI-Powered Virtual Idols
[Picked up by 295 media titles]
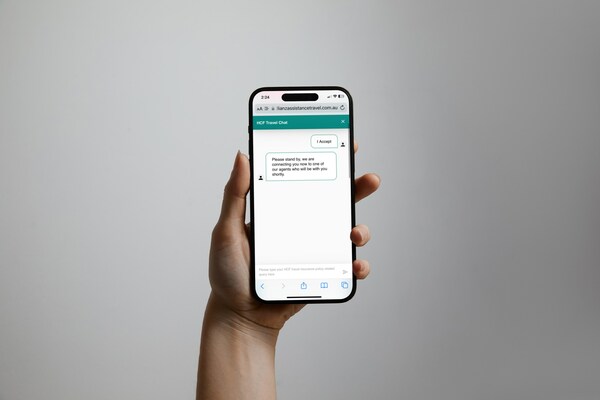
2024-04-12 15:14Allianz Partners Introduces Instant Online Chat for HCF Travel Insurance Customers
[Picked up by 276 media titles]
2024-04-15 08:00Animated Life in Yunnan
[Picked up by 276 media titles]
2024-04-18 14:00