Biotechnology
First Patient Dosed in Clinical Trial of YOLT-101 for the Treatment of FH
SHANGHAI, April 2, 2024 /PRNewswire/ -- YolTech Therapeutics announced that the first patient has been dosed with YOLT-101, the company'sin vivo genome editing candidate being developed as a single dose, potentially curative therapy for Familial Hypercholesterolemia(FH), marking the commencement ...
Origin Agritech Provides First Half Revenue Forecast and Updates Advancements in Hybrids and GMO Development
Estimated Revenue Growth of 30-40% in the First Half of FY 2024 Two New Hybrids Completed National Trials and Four GMO Corn Hybrids Currently in National Trials BEIJING, April 2, 2024 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), a leading Chinese agricultural ...
Lunit to Showcase 7 Studies at AACR 2024: Unveiling AI Innovations in HER2 Expression-Mutation Analysis and CNTN4 Biomarker Identification
- Lunit's AACR 2024 presentations to spotlight HER2 mutation insights and CNTN4's role in immunotherapy success, pioneering next-gen personalized treatment approaches, supported by the AI-powered Lunit SCOPE suite SEOUL, South Korea, April 1, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading...
Aculys Pharma Announces Appointment of Hidemasa Tanigaki as New CEO
~Hidemasa's appointment bolsters Aculys' position as a leader in the field of CNS inJapan and Asia~ TOKYO, April 1, 2024 /PRNewswire/ -- Aculys Pharma, Inc. ("Aculys"), a clinical-stage biopharmaceutical company focused on commercializing innovative treatments for neurological conditions, today ...
LISCure Biosciences receives U.S. FDA Fast Track designation for LB-P8 for the treatment of primary sclerosing cholangitis (PSC)
* Phase 2 study is underway and LB-P8 is the only live biotherapeutic product currently reported to be in clinical development for the treatment of PSC * FDA's Fast Track designation for LB-P8 underlines the urgent need for treatment options to fulfill the unmet medical needs of people affecte...
73% CNS ORR! FDA Granted ODD to Utidelone Injectable (UTD1) from Biostar Pharma for the Treatment of Breast Cancer Brain Metastasis
SAN FRANCISCO, March 29, 2024 /PRNewswire/ -- Biostar Pharma, Inc., the U.S. subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. which is a synthetic biology driven biopharma company focusing on the development and commercialization of innovative oncology drugs, announced today that their cor...
Alebund's Innovative Investigational Drug AP303 Receives FDA Orphan Drug Designation (ODD) for the Treatment of Autosomal Dominant Polycystic Kidney Disease (ADPKD)
SHANGHAI, March 29, 2024 /PRNewswire/ -- Alebund Pharmaceuticals
IASO Bio Announces NMPA's IND Approval for Equecabtagene Autoleucel in Second- and Third-Line Treatment of Multiple Myeloma
SHANGHAI and NANJING, China and SAN JOSE, Calif., March 29, 2024 /PRNewswire/ -- IASO Bio, a biopharmaceutical company engaged in discovering, developing, manufacturing and marketing innovative cell therapies and antibody products, today announced thatChina National Medical Products Administratio...
Jacobio Pharma Announces 2023 Annual Results
BEIJING, SHANGHAI and BOSTON, March 28, 2024 Jacobio/PRNewswire/ -- Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced its 2023 annual results. The revenue wasRMB63.5 million, the R&D investment wasRMB372 million, the cash and cash equivalent at...
Bridge Biotherapeutics Launches a Research Collaboration with Emory University School of Medicine to Explore Combination Therapy of BBT-877 for KRAS/P53 Mutant NSCLC Patients Resistant to Anti-PD-1 Blockade
SEONGNAM, South Korea and ATLANTA, March 28, 2024 /PRNewswire/ --
Bridge Biotherapeutics (KQ288330)
Harbour BioMed Reports Full Year 2023 Financial Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, March 28, 2024 /PRNewswire/ -- Harbour BioMed ("HBM" or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development and commercialization of novel antibody therapeutics focusing on immune-oncol...
GenFleet and BeiGene Enter into Trial Collaboration for a Potentially First-in-class Combination Therapy to Initiate Phase Ib/II Study of GFH009 (CDK9 inhibitor) and BRUKINSA® (zanubrutinib) Treating Diffuse Large B Cell Lymphoma
SHANGHAI, March 28, 2024 /PRNewswire/ -- GenFleet Therapeutics, a clinical-stage biotechnology company focusing on cutting-edge therapies in oncology and immunology, today announced it has entered into a clinical trial collaboration and supply agreement with BeiGene Switzerland GmbH to start a c...
Tigermed Reports Full Year 2023 Results
HANGZHOU, China, March 28, 2024 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. ("Tigermed" or the "company") (Stock code: 300347.SZ / 3347.HK), a leading global provider of integrated research and development solutions for biopharmaceutical and medical device industry, announced its annua...
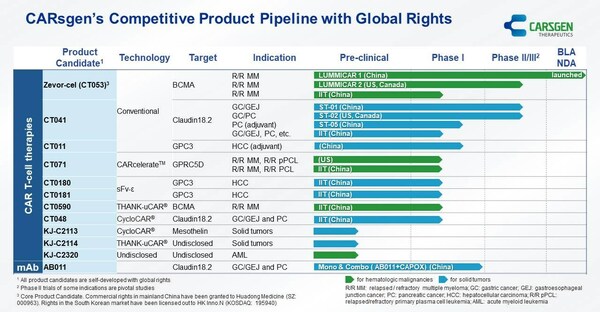
CARsgen Announced 2023 Annual Results
SHANGHAI, March 27, 2024 /PRNewswire/ -- March 27, 2024, CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced its 2023 Annual Results. Business Highlights * Z...
YolTech Therapeutics to Participate in 2024 Cell & Gene Meeting on the Mediterranean
SHANGHAI, March 27, 2024 /PRNewswire/ -- YolTech Therapeutics, a trailblazing biopharmaceutical company specializing in gene editing, is delighted to announce its participation in 2024 Cell & Gene Meeting on the Mediterranean. The event will take place fromApril 9th to 11th, 2024, at the esteemed...
Sanyou Bio: Sanyou's Intelligence-Enabled Innovative Biologics R&D Platform Leads in the New Era of Biological Drug Development
SHANGHAI, March 27, 2024 /PRNewswire/ -- Dr. Guojun Lang, founder and CEO of Sanyou Biopharmaceuticals provides industry insights: The new era sees the transition towards digitalization and intelligence. Following this trend, we have been thinking and planning the transformative path of digitali...
Bridge Biotherapeutics Announces a Research Collaboration with the University of Colorado School of Medicine to Explore Potential of BBT-877 for Immuno-Oncology
SEONGNAM, South Korea and AURORA, Colo., March 26, 2024 /PRNewswire/ -- Bridge
Biotherapeutics (KQ288330)
WuXi Biologics Reports Solid 2023 Annual Results
Revenue Increased by 11.6% Y-o-Y to RMB17,034.3 Million Gross Profit Rose by 1.5% to RMB6,827.9 Million Non-COVID Revenue Achieved 37.7% Y-o-Y Growth, Strong Momentum Sustained Non-COVID Late-Phase and Commercial Manufacturing Revenue Grew by 101.7% Y-o-Y "R" in CRDMO Thrived with Extended Part...
Lunit SCOPE IO Reveals Promising Results in Neoadjuvant Immunotherapy Study for Head and Neck Cancer Patients
- New research published in Clinical Cancer Research shows significant improvement in distant recurrence-free survival with combined durvalumab and tremelimumab treatment, supported by Lunit SCOPE IO's analysis of tumor microenvironment SEOUL, South Korea, March 26, 2024 /PRNewswire/ -- Lunit (K...
TiumBio Submits CTA for Phase 1b Clinical Trial of TU7710, a Long-acting Recombinant Activated Factor VII, in Hemophilia A or B Patients
* TU7710 has the potential to be a highly effective and long-acting treatment for bleeding episodes as well as for preventing bleeding during surgery or invasive procedures in hemophilia patients with inhibitors * Interim results from an ongoing Phase 1a study in healthy male volunteers will ...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 290 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 289 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00