Medical/Pharmaceuticals
CMUH (Taiwan) Launches "Intelligent Sepsis Early Prediction System (ISEPS)," Detecting Sepsis in One Minute Only
It is the world's first AI-Powered sepsis alert system to save more lives TAICHUNG, Taiwan, April 3, 2024 /PRNewswire/ -- In conventional practice, a blood culture report wouldn't be available until 18 to 72 hours later, rendering it non-feasible to facilitate rapid response. Led by Superintend...
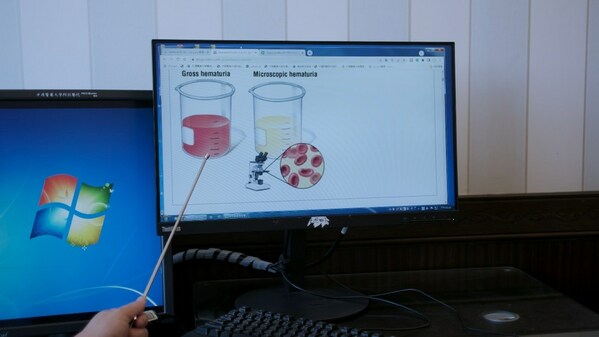
China Medical University Hospital (CMUH) Inventing "Intelligent Urine Sediment Morphology Analysis, IUSMA" to Find Chronic Glomerulonephritis in Just 10 Minutes
TAICHUNG, Taiwan, April 3, 2024 /PRNewswire/ -- The urine sediment examination is primarily used to detect and assess kidney and urinary system health. In the conventional practice, a urine sediment test covers six items: red blood cells, white blood cells, squamous cells, urinary casts, crystals...
YS Biopharma Announces Full Repayment of US$40,000,000 Loan Facility
GAITHERSBURG, Md., April 3, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disea...
Ractigen Announces First Patient Dosed in the Phase I Clinical Trial of RAG-01 for NMIBC
JIANGSU, China, April 3, 2024 /PRNewswire/ -- Ractigen Therapeutics, a pioneer in small activating RNA (saRNA) therapeutics, achieved a major milestone today with the first patient dosed in its First-in-human phase I clinical trial for RAG-01 conducted in collaboration with GenesisCare,Australia'...
Ascletis Announces Strategic Decisions on FXR agonist ASC42
HANGZHOU, China and SHAOXING, China, April 3, 2024 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX:1672, "Ascletis") announces today the strategic decisions on farnesoid X receptor (FXR) agonist ASC42. After thorough analysis of the Phase II trial data of ASC42 for primary biliary cholangitis (PBC) (...
Tianlong Participated in International Medical Congress of SCO Countries in Kyrgyzstan
BISHKEK, Kyrgyzstan, April 3, 2024 /PRNewswire/ -- The International Medical Congress of the SCO countries was held on 27-29 March inBishkek, Kyrgyzstan, under the theme "Innovations and technologies in the development of cardiology and non-communicable diseases in the countries of the Shanghai C...
Human Frontier Science Program: Research Grants Awarded to 108 of the Most Pioneering Life Scientists from 23 Nations
STRASBOURG, France, April 3, 2024 /PRNewswire/ -- Representing only the most daring and truly pioneering life science research, 108 of the world's top scientists from 23 nations have won 2024 Human Frontier Science Program (HFSP) Research Grants. "Excellence isn't enough for us – we support rese...
Great Place To Work unveils Top 10 Best Workplaces in Singapore Healthcare and Biopharma™ 2024
SINGAPORE, April 3, 2024 /PRNewswire/ -- Great Place To Work®, the global
authority on workplace culture announcedSingapore's top 10 Best Workplaces in
Healthcare and Biopharma™
RemeGen's Telitacicept (RC18) Granted Fast Track Designation by United States FDA for Treatment of Primary Sjögren's Syndrome
YANTAI, China, April 3, 2024 /PRNewswire/ -- RemeGen Co. Ltd.
Samyang Holdings's Croquis® Shows New Clinical Cases of PDO thread lift at AMWC 2024
* Sharing Clinical Cases of "Croquis® at AMWC 2024 in Monaco. * Expand market with proven safety and efficacy, 29 countries worldwide. * "Croquis®" Lifting Threads with Samyang' 25 years Expertise in Global Biodegradable Suture Threads. SEOUL, South Korea, April 3, 2024 /PRNewswire/ -- Samy...
Latvian pharmaceutical manufacturing company Grindeks opens new subsidiaries worldwide
RIGA, Latvia, April 3, 2024 /PRNewswire/ -- The leading pharmaceutical company in the Baltics, Grindeks, is developing new products and reinforcing its position not only in European but also global markets. With determination and innovative approaches, Grindeks continues to enhance the competitiv...
TraceLink Named to Fast Company's Annual List of the World's Most Innovative Companies of 2024
TraceLink joins the ranks of Nvidia, YouTube, OpenAI, Novo Nordisk and more BOSTON, April 3, 2024 /PRNewswire/ -- TraceLink has been named to Fast Company 's prestigious list of the World's Most Innovative Companies of 2024. This year's list shines a spotlight on businesses that are shaping their...
GC Cell to Present Multiple Posters at the American Association for Cancer Research (AACR) Annual Meeting 2024
YONGIN, South Korea, April 2, 2024 /PRNewswire/ -- GC Cell
LifeTech Scientific Corporation Announced 2023 Annual Results: International Business Achieved a Robust Growth, Innovation at the Core to Drive the Solid Development
SHENZHEN, China, April 2, 2024 /PRNewswire/ -- LifeTech Scientific Corporation (the "Company" or "Lifetech", Stock code: 1302.HK), a company specializing in minimally invasive interventional medical devices for cardio-cerebrovascular and peripheral vascular diseases, together with its subsidiarie...
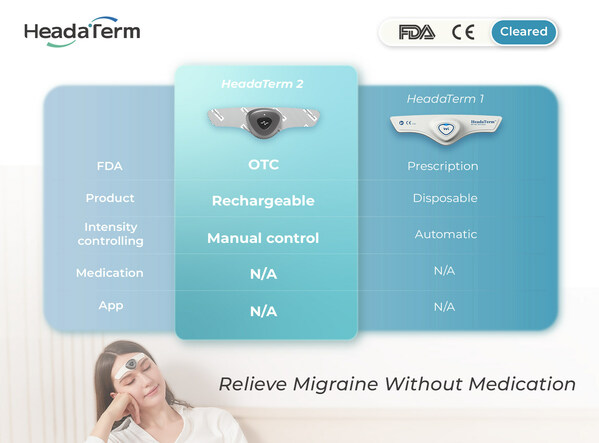
HeadaTerm 2: The Most Affordable FDA Cleared Innovative OTC Anti-Migraine Device
VANCOUVER, BC, April 2, 2024 /PRNewswire/ -- WAT Medical Enterprise achieves a major milestone: HeadaTerm 2 receives the OTC clearance from the U.S. Food and Drug Administration (FDA), making it one of the only wearable anti-migraine devices that is available without a prescription in the U.S. U...
Cognex Launches the World's First 3D Vision System with AI
AI-powered 3D vision system offers fast deployment and reliable inspections for manufacturing automation NATICK, Mass., April 2, 2024 /PRNewswire/ -- Cognex Corporation (NASDAQ: CGNX), the leader in industrial machine vision, today released the In-Sight® L38 3D Vision System, which combines AI, ...
China Medical University Hospital Develops "AISIA" to Diagnose Acute Ischemic Stroke in 90 Seconds to Assist Doctors in Making Medical Decisions
TAICHUNG, Taiwan , April 2, 2024 /PRNewswire/ -- The Neurology Department of China Medical University Hospital (CMUH,Taiwan), teaming up with AI Center at CMUH, has established the AISIA (Artificial Intelligence for Stroke Image Analysis) platform, which contains the "NCCT-based Ischemic Stroke D...
Innovating "Treadmill Exercise Test AI-Assisted Interpretation System," CMUH(Taiwan) Timely Saves More Patients with Severe Myocardial Infarction
TAICHUNG, Taiwan, April 2, 2024 /PRNewswire/ -- The treadmill exercise test (TET) is an important tool for diagnosing coronary artery disease (CAD), but limited by time spent for the manual interpretation of a dozen charts and the challenges in identifying minor deviations. Traditionally, manual ...
First Patient Dosed in Clinical Trial of YOLT-101 for the Treatment of FH
SHANGHAI, April 2, 2024 /PRNewswire/ -- YolTech Therapeutics announced that the first patient has been dosed with YOLT-101, the company'sin vivo genome editing candidate being developed as a single dose, potentially curative therapy for Familial Hypercholesterolemia(FH), marking the commencement ...
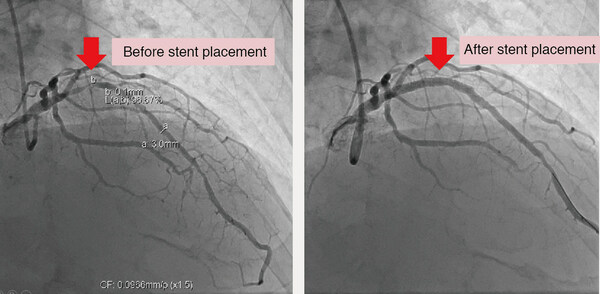
CONCEPT MEDICAL RECEIVES US FDA IDE APPROVAL FOR MAGICTOUCH AVF INDICATION, THEIR FIFTH US CLINICAL STUDY APPROVAL FOR THE MAGICTOUCH PORTFOLIO
TAMPA, Fla., April 2, 2024 /PRNewswire/ -- Concept Medical has been granted 'IDE Approval' from the U.S. Food and Drug Administration (FDA) for MagicTouch AVF, its Sirolimus drug-coated balloon (DCB) catheter, to initiate a clinical study for the treatment of stenotic lesions of Arteriovenous Fis...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 291 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 290 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00