Biotechnology
Antengene Announces XPOVIO® Regulatory Approval in Macau for the Treatment of Relapsed and/or Refractory Multiple Myeloma
* XPOVIO® (selinexor) is the first and only exportin 1 (XPO1) inhibitor approved in Macau. * XPOVIO® has received regulatory approvals in 42 countries and regions including Mainland ofChina, Taiwan China, Hong Kong China, Macau China, South Korea, Singapore and Australia. SHANGHAI AND HONG ...
GC Biopharma's groundbreaking for the plasma fractionation plant in Indonesia
* To commence operation by 2027…with max. 400K liters of plasma production capacity per annum * The first step towards localization of Indonesia's critical medical product YONGIN,South Korea, Dec. 5, 2023 /PRNewswire/ -- GC Biopharma, a South Korean biopharmaceutical company, announced onDece...
Results of three investigator-initiated trials on cadonilimab (PD-1/CTLA-4) for G/GEJC, pMMR/MSS mCRC, and HCC neoadjuvant therapy published at ESMO Asia 2023
First-line therapy for gastric cancer and metastatic colorectal cancer, neoadjuvant therapy for hepatocellular carcinoma HONGKONG, Dec. 5, 2023 /PRNewswire/ -- Akeso (9926.HK) published results from three investigator-initiated trials (IITs) of its bispecific IO drug, cadonilimab (a PD-1/CTLA-4...
inQB8 Medical Technologies LLC and Peijia Medical Limited Report Successful First-in-Human (FIH) Implantation of MonarQ Transcatheter Tricuspid Valve Replacement (TTVR) System in The United States of America
WINCHESTER, Mass. and SUZHOU, China, Dec. 5, 2023 /PRNewswire/ -- inQB8 Medical Technologies LLC (inQB8), in partnership with Peijia Medical Limited (Peijia, (9996.HK)),announced that it has successfully implanted the MonarQ Transcatheter Tricuspid Valve (TTV) in a patient suffering from torrenti...
Ascletis Announces Initiation of Phase III Clinical Trial of ASC40 (Denifanstat) for Treatment of Acne
--The Phase III clinical trial of ASC40 for moderate to severe acne vulgaris will enroll 480 subjects --The protocol of Phase III clinical trial has been agreed by Center for Drug Evaluation, National Medical Products Administration and obtained the approval from Institutional Review Board of Hu...
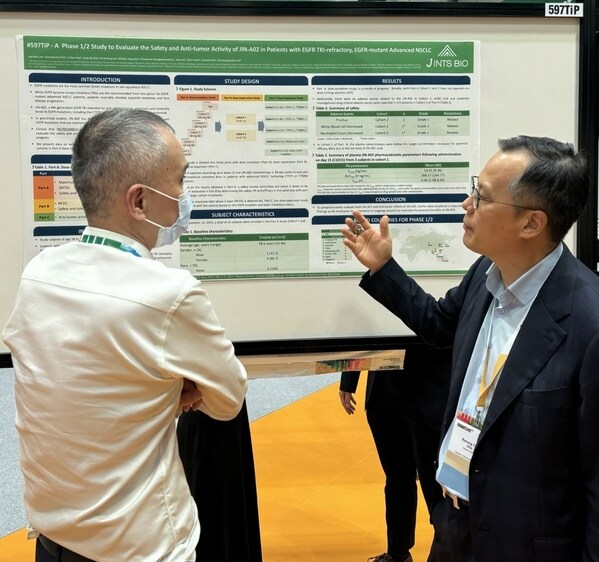
J INTS BIO Gives Presentation of Phase 1/2 study of 'JIN-A02', a Novel Oral 4th Generation EGFR TKI, at ESMO Asia 2023
SEOUL, South Korea, Dec. 5, 2023 /PRNewswire/ -- J INTS BIO announced that
early results of the Phase 1/2 study of its novel, orally administered 4th
generation EGFR-TKI 'JIN-A02' were presented at the ESMO Asia 2023 conference
held inSingapore from 1st to 3rd December 2023.
Clarivate Expands Partnership with VeriSIM Life to Accelerate and De-risk Research and Drug Development
New AI-enabled, integrated workflow provides pharma and biotech companies with comprehensive R&D insights to help minimize late-stage failures during clinical trials LONDON, Dec. 5, 2023 /PRNewswire/ -- Clarivate Plc (NYSE:CLVT), a global leader in connecting people and organizations to intellig...
Insilico Medicine Announces First-in-Human Study for Orally PHD Inhibitor ISM5411 for Inflammatory Bowel Disease (IBD)
* ISM5411 is an oral, PHD-specific inhibitor that facilitates the treatment of IBD by inducing the expression of gut barrier protective genes. * ISM5411 is Insilico Medicine's fifth AI-driven drug program to enter clinical trials, with the first dose of healthy subjects completed inAustralia. ...
CARsgen's CT071 Received IND Clearance from the FDA for Treating Relapsed/Refractory Multiple Myeloma or Relapsed/Refractory Primary Plasma Cell Leukemia
SHANGHAI, Dec. 4, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that the U.S. Food and Drug Administration (FDA) has granted Investiga...
Technoderma Medicines Completes Phase 1 Dose Escalation Clinical Trial of TDM-180935 for Atopic Dermatitis
CHENGDU, China, Dec. 4, 2023 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report that the Company has completed a Phase 1 clinical trial (NCT05525468) of TDM-180935 topical ointment for Atopic Dermatitis (AD). This first cl...
Abbisko Therapeutics Announced the Entry into a Licensing Agreement for Pimicotinib (ABSK021) with Merck
SHANGHAI, Dec. 4, 2023 /PRNewswire/ -- On 4 Dec. 2023, Abbisko Therapeutics announced that it has entered into a licensing agreement with Merck, a leading science and technology company headquartered in Darmstadt,Germany. Under the terms of the agreement, Merck will be granted an exclusive licens...
Mootral introduces new methane reduction technology, doubling efficacy using a well-known natural molecule
Mootral's new platform reduces methane emissions from cows by 50% and can be delivered to both housed and grazing cattle, vastly increasing the opportunity to reduce methane emissions globally LONDON, Dec. 4, 2023 /PRNewswire/ -- Mootral, a British biotech company, announces it has completed the...
Latest Updates of Viva's Portfolio Companies
HONG KONG, Dec. 4, 2023 /PRNewswire/ -- Even with the ever-changing situation, technological innovation is still the most critical component for biopharmaceutical companies' long-term development. This continuous innovation keeps companies up to date and promotes the evolution of R&D and the succ...
Supercell Acquires Exclusive Licensing from Japanese Immunotherapy Leader for Advanced NK Cell Treatment Technology
TAIPEI, Dec. 4, 2023 /PRNewswire/ -- Supercell Biotechnology Corporation commemorates the first anniversary of rebranding from previously-called SinoCell Technologies, due to the company's capital increase by RMT Holding Group. Demonstrating the strategic move at the Healthcare+ Expo Taiwan, the...
Nona Biosciences Announces Collaboration Agreement with Lycia Therapeutics
CAMBRIDGE, Mass., Dec. 3, 2023 /PRNewswire/ -- Nona Biosciences, a global pioneer of technology innovation and antibody discovery and development solutions, announced today it has entered into a collaboration agreement with Lycia Therapeutics, a leader in extracellular protein degradation. Lycia...
Everest Medicines Announces Investigational New Drug Application Acceptance of Zetomipzomib in China
-- Everest plans to contribute to the ongoing global Phase 2b PALIZADE trial of zetomipzomib in active lupus nephritis – -- Zetomipzomib demonstrated clinically meaningful renal responses with a favorable tolerability profile in an earlier Phase 2 trial -- -- Renal and autoimmune diseases are k...
280Bio doses first patient in Phase I clinical trial of TEB-17231, an oral pan-RAS inhibitor in patients with advanced cancers harboring mutations in KRAS, HRAS, or NRAS
SOUTH SAN FRANCISCO, Calif., Dec. 1, 2023 /PRNewswire/ -- 280Bio, Inc., a clinical stage biotechnology company focused on the development of precision oncology medicines, today announced that the first patient was treated with TEB-17231 (YL-17231), a small molecule inhibitor of RAS signaling. 280...
GC Biopharma establishes mRNA production facility in Hwasun, Korea
* Completed the construction of a GMP pilot plant in its Hwasun production plant in Jeollanam-do, Korea * Plans to expand its domain from vaccine development to the field of rare disease treatments by proactively securing mRNA platforms YONGIN, South Korea, Dec. 1, 2023 /PRNewswire/ -- GC Bio...
Innovent Presents Clinical Data Update of IBI351 (KRAS G12C Inhibitor) Monotherapy in Lung Cancer and Colorectal Cancer at ESMO Asia Congress 2023
ROCKVILLE, Md. and SUZHOU, China, Dec. 1, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and...
Cerecin forges strategic partnership with A*STAR's Institute of Molecular and Cell Biology (IMCB) in Singapore to advance R&D in neurological disorders.
Cerecin and IMCB signed a Memorandum of Understanding to collaborate in the areas of neurometabolism, neurodegeneration, metabolic regulators, and brain health and function. SINGAPORE and DENVER, Dec. 1, 2023 /PRNewswire/ -- Cerecin Inc., a clinical-stage biotechnology company at the forefront o...
Week's Top Stories
Most Reposted
Protiviti Transforms Cyber Risk Consulting with CYFIRMA's Advanced Intelligence-Led Cybersecurity
[Picked up by 324 media titles]
2024-04-15 09:00Sephora appoints Xia Ding as Managing Director of Sephora Greater China
[Picked up by 302 media titles]
2024-04-15 17:33AI-DOL: Revolutionising the Future of Entertainment with AI-Powered Virtual Idols
[Picked up by 295 media titles]
2024-04-12 15:14LEGOLAND® School Challenge 2024 Expands Across Asia and Opens for Registration on April 15th
[Picked up by 276 media titles]
2024-04-11 11:00Allianz Partners Introduces Instant Online Chat for HCF Travel Insurance Customers
[Picked up by 275 media titles]
2024-04-15 08:00