Biotechnology
Zhimeng Biopharma will report positive phase 1b trial results on its HBV capsid inhibitor ZM-H1505R in the upcoming International Liver Congress
SHANGHAI, June 14, 2022 /PRNewswire/ -- Shanghai Zhimeng Biopharma announces that it will report the positive results obtained from a phase1b study on its HBV capsid inhibitor ZM-H1505R in the International Liver Congress inJune 22-26, 2022 (the EASL meeting). The recently completed phase 1b stu...
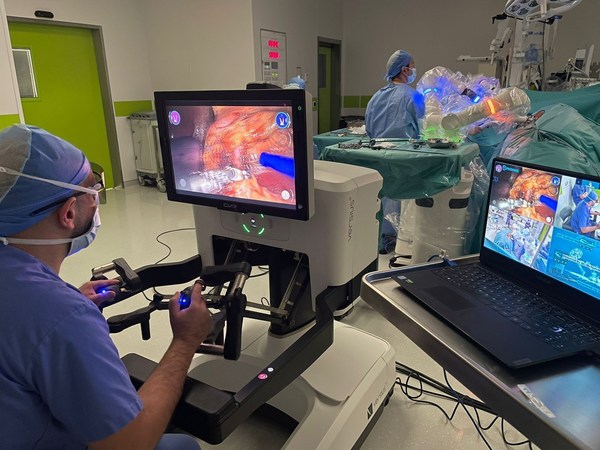
PROXIMIE RAISES $80 MILLION IN SERIES C FUNDING TO ACCELERATE PRODUCT EXPANSION OF FULL-SERVICE CONNECTED SURGICAL PLATFORM
Funding round led by Advent Life Sciences. New investors include Emerson Collective, SoftBank Vision Fund 2, British Patient Capital, Mubadala and the Minderoo Foundation. Proceeds to accelerate development and scale of Proximie's Operating System for the Operating Room - a centralized platform ...
Cellenkos Receives FDA Clearance of Investigational New Drug (IND) Application for CK0804 as Add on Therapy to Ruxolitinib for the Treatment of Myelofibrosis
-- CK0804 represents the company's fourth program to receive IND Clearance -- CK0804 is a partnered program with Incyte HOUSTON, June 14, 2022 /PRNewswire/ -- Cellenkos, Inc., a privately held, clinical stage biotech company that focuses on developing transformative T regulatory cell therapies ...
Kintor Pharma and Etana's Collaboration on Pruxelutamide's COVID-19 Project Awarded with Belt and Road Innovation Project and Fund Support from the Science and Technology Department of Jiangsu Province
SUZHOU, China, June 14, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced that its "clinical trial cooperative research and development and ov...
BIO Asia-Taiwan 2022 kicks off July 27 with theme 'Connecting the Asian Value Chain'
TAIPEI, June 14, 2022 /PRNewswire/ -- During a press event on June 8, 2022, the Taiwan Bio Industry Organization (Taiwan BIO) and the global Biotechnology Innovation Organization (BIO) announced updates toBIO Asia–Taiwan 2022 Onsite + Online International Conference and Exhibition, to be held in ...
Innovent and Etana Jointly Announce the Approval of Bevagen® (Bevacizumab Biosimilar) by the Indonesian Food and Drugs Authority (BPOM)
SAN FRANCISCO and SUZHOU, China, June 14, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and...
Treadwell Announces Initiation of Patient Dosing in TWT-203, a Phase 1b/2 study of TTK Inhibitor, CFI-402257, in Patients with ER+/Her2- Breast cancer
NEW YORK and HONG KONG, June 14, 2022 /PRNewswire/ -- Treadwell Therapeutics, a clinical-stage biotechnology company developing novel medicines for unmet needs in cancer, today announced the initiation of patient dosing in TWT-203, its Phase1b/2 study to evaluate CFI-402257, an oral, best-in-clas...
Good News from Taiwan Participants in BIO 2022 - Pharmasaga and PrecisemAb Make It to the Start-Up Stadium Finalists Defeating Hundred Teams
TAIPEI, June 13, 2022 /PRNewswire/ -- As the post-pandemic era approaches, the biomedical industry has become a strategic domain of focal emphasis inTaiwan and would be the critical impetus for the next wave of growth. In order to step up international connection and strive for business opportuni...
Dompé Announces Results of Phase 2 Study Evaluating the Efficacy and Safety of Reparixin in Patients with Severe COVID-19 Pneumonia
* In hospitalized patients with COVID-19 Pneumonia, the rates of clinical events among those treated with Reparixin (n=36) were statistically significantly lower than standard of care (n=19). * The study is the first evaluation of the efficacy and safety of Reparixin an IL-8 inhibitor in pati...
Novavax COVID-19 Vaccine Nuvaxovid™ Provisionally Registered in Australia as a Booster in Individuals Aged 18 and Over
* In Australia, Nuvaxovid™ is the first protein-based COVID-19 vaccine registered for use as a booster regardless of previous vaccine history GAITHERSBURG, Md., June 13, 2022 /PRNewswire/ -- Novavax, Inc. (NASDAQ: NVAX), a biotechnology company dedicated to developing and commercializing next-...
POSITIVE DATA FOR PAXALISIB IN TWO CHILDHOOD BRAIN CANCERS PRESENTED AT 20TH ISPNO ANNUAL MEETING
SYDNEY, June 13, 2022 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce the presentation of new data regarding the activity of paxalisib in two forms of childhood brain cancer with poor prognosis and limited t...
Curocell announced impressive CR rate in Phase 1 study with anbal-cel, the next generation CD19 CAR-T integrated OVIS™ platform
- 82% ORR (9 of 11 patients) and 82% (9 of 11 patients) CR rate documented after anbal-cel treatment to the patients in relapsed/refractory large B-cell lymphoma - 2 out of 3 patients dosed with 2x105 cells/kg of anbal-cel lasting the complete response for more than 12 months - Well tolerat...
Ascletis Announces Appointment of Mr. John P. Gargiulo, Former North America President and CEO of Daiichi Sankyo, as Chief Business Officer
HANGZHOU, China and SHAOXING, China, June 13, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis"), today announces the expansion of management team with appointment of Mr.John P. Gargiulo, former North America President and CEO of Daiichi Sankyo, as Chief Business Officer. Mr.John ...
Innovent and IASO Bio Present Updated Data of BCMA CAR-T Cell Therapy (Equecabtagene Autoleucel) at EHA 2022
SAN FRANCISCO and SUZHOU, China, June 13, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology and...
Innovent Releases Results of High-dose Cohorts in Phase 1 Clinical Study of Mazdutide (IBI362) in Chinese Adults with Overweight or Obesity at ENDO 2022
SAN FRANCISCO and SUZHOU, China, June 13, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and...
EHA 2022 | Ascentage Pharma Releases Encouraging Results of Bcl-2 Inhibitor Lisaftoclax (APG-2575) in Chinese Patients with Relapsed/Refractory Non-Hodgkin Lymphoma
SUZHOU, China, and ROCKVILLE, MD, June 12, 2022 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that it has released results from a Phase I study ...
Everest Medicines Announces Approval of Trodelvy® in China for Second-Line Metastatic Triple-Negative Breast Cancer
– Trodelvy is Everest's First Product to Receive Regulatory Approval in China – – Everest Plans to Launch Commercial Sales of Trodelvy in China in Q4 2022 – – Conference call will be held to discuss the approval– SHANGHAI, June 10, 2022 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", o...
RAD adds brain tumor technology to portfolio
* Sublicensing of promising imaging & therapeutic radiopharmaceutical from leading US university,Case Western Reserve University (CWRU), Ohio * PTPµ (PTPmu), the target, is a unique biomarker present only in tumor cells but not healthy cells * The radionuclide carrying PTPµ-targeting agent h...
BIORCHESTRA, presenting lead candidate BMD-001 for Alzheimer's disease and drug delivery system technology at the 2022 BIO International Convention
BOSTON, June 9, 2022 /PRNewswire/ -- BIORCHESTRA Co., Ltd (BIORCHESTRA), is scheduled to give a company presentation onJune 16, 12:00 at BIO International convention, taking place inSan Diego, California, between June 13-16, 2022. BIORCEHSTRA's company presentation will cover its lead therapeut...
Brii Biosciences Announces Positive Data from a Randomized, Single-Blind Study of its Long-Acting COVID-19 Neutralizing Antibody Therapy, Amubarvimab/Romlusevimab Combination, in China
Data are consistent with the results of the global Phase 3 ACTIV-2 trial, demonstrating a favorable safety and tolerability profile in people with both severe and non-severe SARS-CoV-2 infections inChina Clinical benefits were observed in efficacy indicators such as RNA conversion rate, resoluti...
Week's Top Stories
Most Reposted
Protiviti Transforms Cyber Risk Consulting with CYFIRMA's Advanced Intelligence-Led Cybersecurity
[Picked up by 324 media titles]
2024-04-15 09:00Sephora appoints Xia Ding as Managing Director of Sephora Greater China
[Picked up by 309 media titles]
2024-04-15 17:33With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 281 media titles]
2024-04-18 21:26Animated Life in Yunnan
[Picked up by 278 media titles]
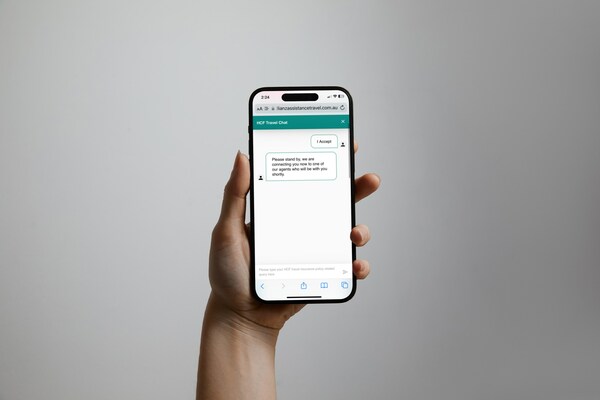
2024-04-18 14:00Allianz Partners Introduces Instant Online Chat for HCF Travel Insurance Customers
[Picked up by 276 media titles]
2024-04-15 08:00