Medical/Pharmaceuticals
2020 Global Survey from ISAPS Sees Significant Changes in Aesthetic Procedures During Pandemic
COVID-19 practice closures lead to a 10.9% decrease in surgical procedures WEST LEBANON, N.H., Dec. 28, 2021 /PRNewswire/ -- The International Society of Aesthetic Plastic Surgery (ISAPS) released the results of its annual Global Survey on Aesthetic/Cosmetic Procedures today, which showcased the ...
EOFlow and Zihipp Announce Joint Venture for Development of Innovative Solutions for Treatment of Obesity & NASH
* Establishment of Joint Venture Company named SanPlena in the US * Drug-device combination of EOFlow's smart wearable drug delivery platform and novel peptide derivatives developed by a team led by Prof. SirStephen Bloom , world-renowned researcher in the field of obesity and metabolism at Imp...
Muslim-Friendly Malaysia Showcases Healthcare Excellence at Expo 2020 Dubai
DUBAI, UAE, Dec. 28, 2021 /PRNewswire/ -- Malaysia Healthcare, will be showcasing its excellence in healthcare service delivery and providing seamless healthcare journey experiences during Week 14 of Expo 2020 Dubai. From 2nd - 8th January 2022, expo participants can visit the Malaysia Pavilion to...
Akeso's IL-17A MONOCLONAL ANTIBODY (GUMOKIMAB) COMPLETION OF PATIENT ENROLLMENT IN PHASE II CLINICAL TRIAL FOR THE TREATMENT OF ANKYLOSING SPONDYLITIS
HONG KONG, Dec. 27, 2021 /PRNewswire/ -- Today, Akeso, Inc. (09926.HK) announces that Gumokimab (IL-17A monoclonal antibody, AK111), an innovative drug independently developed by the Company for the treatment of active ankylosing spondylitis has been completed. Such clinical trial aims to evalua...
Regor Therapeutics Announces U.S. FDA Authorization to Conduct Regor's First-in-Human Clinical Trial with the Next Generation Targeted Inhibitor RGT-419B for Oncology
SHANGHAI, Dec. 27, 2021 /PRNewswire/ -- On December 23, Regor Therapeutics, a clinical-stage biotech company,announced authorization from the US Food and Drug Administration (FDA) to proceed with Regor's Phase 1 clinical development plans for RGT-419B. RGT-419B is a new generation CDK2/4/6, smal...
CStone announced new drug approval of precision therapy AYVAKIT® (avapritinib) in Hong Kong, China for the treatment of PDGFRA D842V mutant gastrointestinal stromal tumors (GIST)
* AYVAKIT is the first precision therapy approved in Hong Kong, China for the treatment of patients with PDGFRA D842V mutant GIST * This is CStone's fifth NDA approval in Greater China this year SUZHOU, China ,Dec. 28, 2021 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), a leadi...
Lunit Obtains MDSAP Certificate, Granted Fast-Track Regulatory Process in Major Countries
* Lunit meets MDSAP requirements as a global standard medical AI software manufacturer * QMS audit exemption in the US, Canada, Japan, Australia, and Brazil SEOUL, South Korea, Dec. 27, 2021 /PRNewswire/ -- Lunit, a leading AI software company for cancer screening and treatment, announced it ...
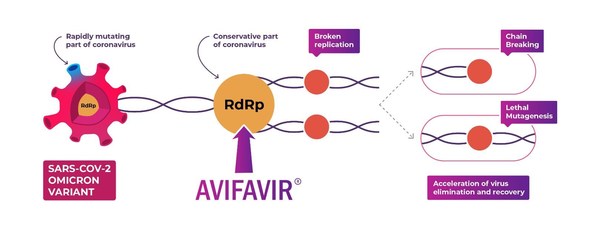
ChemRar Group announces the Russian Avifavir® drug is effective against variants of COVID-19, including Delta and Omicron
Avifavir® is effective against various variants of coronavirus, including Delta and Omicron, as it affects the highly conservative and mutation-resistant replication systems of RNA virus (RdRp). The virus is incapable of developing resistance to favipiravir even with long-term exposure on infect...
I-Mab Announces IND Approval from China NMPA for Phase 2 Clinical Trial of Enoblituzumab in Combination with Pembrolizumab in Solid Tumors
SHANGHAI and GAITHERSBURG, Md., Dec. 27, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, announced that the Center for Drug Evaluation (CDE) ofChina's National ...
Kintor Pharma Provides Update on One of its Three Multi-Regional Phase 3 Trials of Proxalutamide for COVID-19
SUZHOU, China, Dec. 27, 2021 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, today provided an update on its multi-regional study of proxalutamide for the treatme...
Brii Bio Doses First Patient in Phase 2a/2b Clinical Trial of BRII-179 (VBI-2601) for the Functional Cure of Chronic Hepatitis B
DURHAM, N.C. and BEIJING, Dec. 27, 2021 /PRNewswire/ -- Brii Biosciences
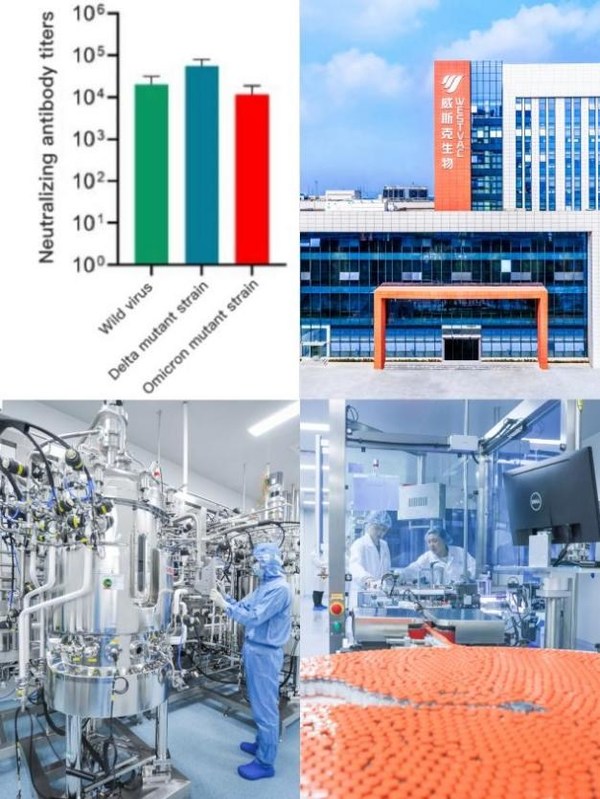
COVID-19 Vaccine Developed by WestVac BioPharma Has High Titer Neutralizing Antibodies Against Omicron
CHENGDU, China, Dec. 24, 2021 /PRNewswire/ -- WestVac BioPharma Co., Ltd. has achieved a significant progress in the second generation COVID-19 vaccine against the Omicron mutant strain. The vaccine belongs to the latest fifth generation vaccine technology, targeting the S-RBD protein of the COV...
Innovent Announces NMPA of China Acceptance of a Supplemental New Drug Application for TYVYT® (Sintilimab Injection plus Bevacizumab Biosimilar Injection and Chemotherapy in Patients with EGFR-mutated Non-squamous Non-small Cell Lung Cancer who Progressed after EGFR-TKI Therapy
SAN FRANCISCO and SUZHOU, China, Dec. 24, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Laekna Presents Vision and Strategy at NewYorkBIO/NYSE Emerging Company Showcase
SHANGHAI and WARREN, N.J., Dec. 24, 2021 /PRNewswire/ -- Laekna was invited by NewYorkBIO and the New York Stock Exchange (NYSE) to present, as an emerging biotech company at the "Emerging Company Showcase" that was held earlier this month inNew York. Dr. Guy Rosenthal, Vice President, Head of C...
Convidecia™ Phase III Results Published in The Lancet
* 96.0% effective against severe disease 14 days post-vaccination for population aged 18 and above. * Single-dose vaccine received approvals in at least 10 markets including China, Mexico, Ecuador, Chile, Argentina, Hungary, Kirghizstan, Pakistan, Indonesia and Malaysia. TIANJIN, China, Dec. ...
Novavax and SK bioscience Expand Manufacturing Agreement
- Novavax secures additional manufacturing capacity through 2022 - SK bioscience secures long-term license to supply NVX-CoV2373 for the Korean market GAITHERSBURG, Md., Dec. 24, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializin...
Novo Nordisk BASEA office certified Best Place to Work for 2021
KUALA LUMPUR, Malaysia, Dec. 23, 2021 /PRNewswire/ -- Novo Nordisk BASEA office, part of the global healthcare company with more than 95 years of innovation and leadership in diabetes care, has recently achieved the Best Place to Work certification for 2021. During the assessment, the organizati...
Curocell, Breaks Ground on 'CAR-T Manufacturing GMP Facility'
DAEJEON, South Korea, Dec. 23, 2021 Curocell, currently on PhaseⅠCAR-T Therapy
clinical trial withCRC01 (anbalcabtagene autoleucel) has recently broken ground
for the new CAR-T Center in Dungok Residential & Industrial Area in Daejeon
International Science & Business Belt.
Ng Teng Fong Charitable Foundation Encourages COVID-19 Vaccination Amongst Elderly Citizens
* 200 Lucky Draw Winners Received Elderly Prize * Over 50,000 Gift Packs Being Donated to Vaccinated Elderly HONG KONG, Dec. 23, 2021 /PRNewswire/ -- Ng Teng Fong Charitable Foundation Limited ('NTFCF'), the philanthropic arm of Sino Group (the 'Group'), encourages COVID-19 vaccination among ...
Luye Pharma Releases Top-Line Results from a Phase II Clinical Trial of Its New Antidepressant Ansofaxine Hydrochloride Extended-Release Tablets
CHONGQING, China, Dec. 23, 2021 /PRNewswire/ -- Luye Pharma Group today released the encouraging top-line results from a Phase II clinical trial of its new antidepressant, Ansofaxine Hydrochloride Extended-Release Tablets (LY03005), at the 19th National Psychiatry Conference of the Chinese Medica...
Week's Top Stories
Most Reposted
Protiviti Transforms Cyber Risk Consulting with CYFIRMA's Advanced Intelligence-Led Cybersecurity
[Picked up by 324 media titles]
2024-04-15 09:00Sephora appoints Xia Ding as Managing Director of Sephora Greater China
[Picked up by 309 media titles]
2024-04-15 17:33AI-DOL: Revolutionising the Future of Entertainment with AI-Powered Virtual Idols
[Picked up by 295 media titles]
2024-04-12 15:14Animated Life in Yunnan
[Picked up by 276 media titles]
2024-04-18 14:00Allianz Partners Introduces Instant Online Chat for HCF Travel Insurance Customers
[Picked up by 276 media titles]
2024-04-15 08:00