Pharmaceuticals
Mabwell to Present Clinical Trial Data of 2 Programs at ESMO Congress 2023
SHANGHAI, July 25, 2023 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that clinical trial data from phase I/II study for advanced solid tumor of Nectin-4-targeting ADC (9MW2821), and phase III study of Long-Acting Recombinant Hu...
RemeGen's Telitacicept Shows Significant Promise in Phase II Trial for Primary Sjogren's Syndrome: Findings Published in Prestigious Rheumatology Journal
YANTAI, China, July 25, 2023 /PRNewswire/ -- RemeGen Co., Ltd.
Foresee Pharmaceuticals Selected for Late-Breaking Oral Presentation on its Aderamastat Phase 2 Study Results at the European Respiratory Society (ERS) International Congress 2023
TAIPEI, July 25, 2023 /PRNewswire/ -- Foresee Pharmaceuticals (TPEx: 6576), ("Foresee") announced today that the company has been selected for a late-breaking oral presentation on the positive primary outcomes of its aderamastat (FP-025) Phase 2 proof-of-concept allergic asthma study at the Euro...
Clinical Results of ASC22 (Envafolimab) in Combination with Chidamide for Functional Cure of HIV Infection Presented at the 12th IAS Conference on HIV Science
HANGZHOU and SHAOXING, China, July 25, 2023 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces that Shanghai Public Health Clinical Center presented clinical results of ASC22 (Envafolimab) in combination with Chidamide for functional cure of human immunodeficiency virus...
Mabwell Announces the NMPA Approval of Novel B7-H3 ADC (7MW3711) for IND
SHANGHAI, July 21, 2023 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its clinical trial application of 7MW3711 for advanced malignant solid tumor was approved by the National Medical Products Administration (NMPA). 7MW3711...
Menarini Group Receives Positive CHMP Opinion Recommending EC Approval of ORSERDU® (Elacestrant) for the Treatment of Patients with ER+, HER2- Locally Advanced or Metastatic Breast Cancer with an Activating ESR1 Mutation
* Each year in Europe more than 550,000 patients are diagnosed with breast cancer, of whom 70% have estrogen receptor (ER)-positive disease¹; more than 147,000 breast cancer patients inEurope die annually from the disease² * If approved by the European Commission, ORSERDU would be the first an...
SunHo Announces First Patient Dosed in Phase I/II Clinical Trial of a Potential First-in-class Immunocytokine IBB0979
NANJING, China, July 21, 2023 /PRNewswire/ -- SunHo BioPharmaceutical Co., Ltd. ("SunHo"), a clinical-stage leading biopharmaceutical company in immunocytokines with full-set of capabilities from discovery to commercialization, announced that IBB0979 (B7H3-IL10 immunocytokine), a potential first...
GC Biopharma's "GC FLU" obtains vaccine approval in Egypt: First-ever approval of the company's quadrivalent flu vaccine on the African continent
* Striving to further expand its territory to Africa and the Middle East from Southeast Asia and Latin America * "Will create synergy between international procurement market and private markets of individual countries so as to boost sales and profitability" YONGIN, South Korea, July 21, 2023...
Jacobio Pharma to Present Data of Glecirasib in Combination with JAB-3312 at the 2023 ESMO Conference
BEIJING and SHANGHAI and BOSTON, July 20, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company focusing on undruggable targets, is pleased to announce today that the company will present its clinical results in the form of Proffered Paper presentation at the 2023 Europ...
Saphnelo now available in Malaysia as an add-on treatment for adult patients with moderate to severe, active autoantibody-positive systemic lupus erythematosus (SLE)
KUALA LUMPUR, Malaysia, July 21, 2023 /PRNewswire/ -- AstraZeneca's Saphnelo ( anifrolumab) has now become available as add-on treatment for adult patients in Malaysia with moderate to severe, active autoantibody-positive systemic lupus erythematosus (SLE) following insufficient response to sta...
US FDA Grants Orphan Drug Designation of SN Bioscience's Nano Anti-Cancer Drug 'SNB-101' for Small Cell Lung Cancer
SEONGNAM, South Korea, July 20, 2023 /PRNewswire/ -- SN Bioscience Co. Ltd. (CEO Park Young-hwan) announced onJuly 19 that the US FDA had granted an orphan drug designation for small cell lung cancer for SNB-101 (API: SN-38), a new polymer nanoparticle drug under development. SNB-101 is the world...
Lynk Pharmaceuticals Announces First Atopic Dermatitis Patient Dosed with LNK01004 in Phase Ib Clinical Study
HANGZHOU, China, July 21, 2023 /PRNewswire/ -- Lynk Pharmaceuticals Co., Ltd. (hereinafter referred to as 'Lynk Pharmaceuticals'), an innovative clinical stage company, announced that it has dosed the first patient with atopic dermatitis in a Phase Ib clinical trial of its innovative drug LNK0100...
Viva Biotech's Portfolio Company Riparian Pharmaceuticals Announces Exclusive License Agreement and Research Agreement with Pfizer for Novel Cardiovascular Programs
WATERTOWN, Mass., July 20, 2023 /PRNewswire/ -- Riparian Pharmaceuticals, a Viva Biotech portfolio company, is a biotechnology company focused on discovering novel therapeutics for cardiovascular diseases. Riparian today announced it has entered into an exclusive license agreement and research a...
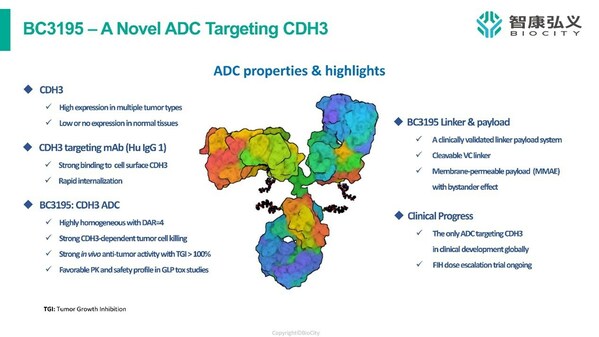
BioCity announces the first patient dosed with its first-in-class CDH3-targeting ADC BC3195 in a Phase 1 Trial
WUXI, China, July 19, 2023 /PRNewswire/ -- BioCity Biopharma, a clinical-stage biopharmaceutical company committed to developing novel and highly differentiated, modality-independent therapeutics for cancer and autoimmune disorders, today announced the dosing of the first patient in a Phase 1a/1b...
Biotheus Announces Strategic Research Collaboration and Worldwide License Agreement with BioNTech
ZHUHAI, China, July 19, 2023 /PRNewswire/ -- Biotheus Inc. ("Biotheus"), a clinical-stage biotech company dedicated to the discovery and development of biologics for oncology and inflammatory diseases, announced today that it has entered into a strategic research collaboration, option and worldwi...
Gannex Announces the Completion of Patient Enrollment for Phase II Clinical Trial of ASC42, an FXR Agonist, for Primary Biliary Cholangitis
-- Gannex is expected to release topline data of the Phase II clinical trial by the end of 2023 SHANGHAI, July 20, 2023 /PRNewswire/ -- Gannex Pharma Co., Ltd. ("Gannex"), a wholly-owned company of Ascletis Pharma Inc. (HKEX:1672) announces today the completion of enrollment of 98 patients with ...
Telix Reports Continued Double-Digit Revenue Growth and Positive Operating Cash Flow
MELBOURNE, Australia, July 19, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today issues its Appendix 4C quarterly cash flow report and accompanying Activities Report for the quarter ended30 June 2023 (Q2 2023). All figures are in AUD$ unless otherwise stated[...
The International Conference on Pharmaceutical Innovation and Development 2023 Kicked off in Yantai
YANTAI, China, July 19, 2023 /PRNewswire/ -- On the morning of July 13, the International Conference on Pharmaceutical Innovation and Development 2023 kicked off in Yantai. The conference was hosted by the People's Government of Shandong Province, co-sponsored by the Chinese Pharmaceutical Associa...
Yikaida CAR-T Cell Therapy Approved as a Second-line Therapy for New Indication
SHANGHAI , July 18, 2023 /PRNewswire/ -- The product of Fosun Kite Biotechnology Co., Ltd., jointly established by Shanghai Fosun Pharmaceutical (Group) Co., Ltd. and Kite Pharma ofthe United States (trade name: Yikaida®) for the treatment of adult large B-cell lymphoma (r/r LBCL) ("this new ind...
Daewoong Pharmaceutical has obtained approval for SGLT2i + metformin combination drug Envlomet
SEOUL, South Korea, July 19, 2023 /PRNewswire/ -- Daewoong Pharmaceutical obtained approval for a SGLT-2 inhibitor+metformin combination drug in just one month after the release of Envlo Tab., the 1st SGLT-2 inhibitor by Korea, expanding its product portfolio. Daewoong Pharmaceutical (Jeon Sen...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 290 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 289 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00