Pharmaceuticals
Hummingbird Bioscience Announces HMBD-002 Trials in Progress Poster at ASCO Annual Meeting 2023
* HMBD-002, a non-depleting, high-affinity anti-VISTA antibody, possesses key design features enabling robust anti-tumor activity in preclinical models, positioning it as a potentially important new therapy for VISTA-expressing cancers including triple-negative breast cancer and non-small cell ...
AMP945 Combined with FOLFIRINOX Enhances Treatment Effects in model of Pancreatic Cancer
HIGHLIGHTS * Amplia's highly selective FAK inhibitor AMP945, when used in combination with FOLFIRINOX, enhances survival in a preclinical model of pancreatic cancer * FOLFIRINOX is the most widely used treatment for pancreatic cancer patients in the US,Canada and most European countries * T...
APPLICATION OF HOSPITAL-BASED HEALTH TECHNOLOGY ASSESSMENT: EXPERIENCE FROM THE WORLD AND SITUATION IN VIETNAM
HO CHI MINH CITY, Vietnam, May 29, 2023 /PRNewswire/ -- In the limited medical sources circumstance, the technology evaluation will help policymakers choose and establish the most appropriate health technology coordinating with conditions and actual occurrences, thereby ensuring the best medical ...
Harbour BioMed Reports Results of Phase Ib Clinical Trial of Porustobart in Combination of Toripalimab in Patients with Hepatocellular Carcinoma at ASCO 2023
* Porustobart in combination of toripalimab showed promising anti-tumor activity * Porustobart in combination of toripalimab showed acceptable safety profile in HCC * Porustobart in combination of toripalimab showed favorable PK/PD signature CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZH...
77.4% ORR! Abbisko to present the updated clinical phase Ib data of Pimicotinib (ABSK021) at the 2023 ASCO Annual Meeting
SHANGHAI, May 28, 2023 /PRNewswire/ -- Abbisko Therapeutics Co., Ltd. ("Abbisko Therapeutics" hereafter) today announced that the updated results of Phase Ib study of its CSF-1R inhibitor Pimicotinib (ABSK021) in treating patients with advanced tenosynovial giant cell tumor ("TGCT"), will be rele...
Menarini Group Shares New Analysis from EMERALD Clinical Study of ORSERDU® (Elacestrant) in Metastatic Breast Cancer at ASCO 2023
* ORSERDU (elacestrant) was approved by the FDA in January 2023 for estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer withESR1-mutations (ESR1-mut) that are found in up to 40% of tumors * In patients whose tumors har...
ProfoundBio Announces Further Advancement of Lead Programs - PRO1184 (Rinatabart Sesutecan) and PRO1160 in the Clinic
* PRO1160 first patient dosed in the US * China NMPA clearance for PRO1184 (rinatabart sesutecan, Rina-S) and PRO1160 to initiate clinical trials * Rina-S preliminary clinical data is encouraging SUZHOU, China and WOODINVILLE, Wash., May 26, 2023 /PRNewswire/ -- ProfoundBio, a clinical-stage...
FivepHusion Announces Strategic Collaboration with Treehill Partners and Syneos Health
Supporting FivepHusion's Global Development and Commercialization Strategy SYDNEY, May 26, 2023 /PRNewswire/ -- FivepHusion, an advanced clinical-stage biotechnology company, today announced its collaboration with Treehill Partners, aNew York-based strategic and financial advisory firm specializi...
Ascentage Pharma Presents Updated Results from Multiple Clinical Studies at American Society of Clinical Oncology Annual Conference
SUZHOU, China, and ROCKVILLE, Md., May 25, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, chronic hepatitis B (CHB), and age-related diseases, announced today that 4 of its abstracts were selected for presenta...
Antengene To Present Latest Results from TORCH-2 Study of ATG-008 in Advanced Solid Tumors in Poster Discussion at ASCO 2023
* The TORCH-2 study is a Phase I/II trial of the mTORC1/2 inhibitor ATG-008 plus the Anti-PD-1 monoclonal antibody toripalimab for the treatment of patients with advanced solid tumors * The combination treatment produced an objective response rate (ORR ) of 52.4% in the advanced cervical canc...
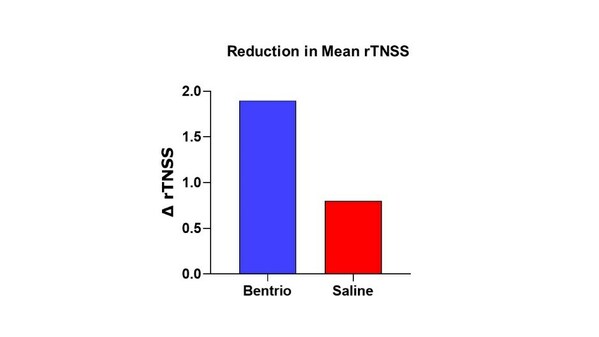
Altamira Therapeutics Reports Positive Top-Line Data from Bentrio Clinical Trial in Seasonal Allergic Rhinitis
* Bentrio® meets primary efficacy endpoint in NASAR clinical trial in seasonal allergic rhinitis * Clinically relevant and statistically significant improvement in Total Nasal Symptom Score over saline nasal spray control (p = 0.012) * Bentrio efficacy and tolerability rated as "good" or "ve...
Dizal's Oncology Pipeline Continues to Impress with Two Oral Presentations at 2023 ASCO Annual Meeting
SHANGHAI, May 25, 2023 /PRNewswire/ -- Dizal (688192.SH) today announced that data from its oncology portfolio will be presented at the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting,June 2-6, 2023 in Chicago. The data which include updated analyses of Dizal's two leading assets...
Daewoong Pharmaceutical Publishes Molecular Mechanism of Bersiporocin as an Antifibrotic in EMBO Molecular Medicine
* Elucidated molecular mechanism behind safety and efficacy of Bersiporocin * Bersiporocin is currently in Phase II clinical trial for Idiopathic Pulmonary Fibrosis SEOUL, South Korea, May 25, 2023 /PRNewswire/ -- Daewoong Pharmaceutical (CEOLee Chang-jae and Jeon Sengho) announced on the 25th...
I-Mab Announces Encouraging Phase 1b/2 Study Results of Patients with Advanced NSCLC Receiving Uliledlimab and Toripalimab Combination Therapy at ASCO 2023
- Uliledlimab is a differentiated monoclonal antibody designed to target CD73 and promote stronger activation ofpatients' immune system against cancer cells. - In newly diagnosed patients who were not eligible for or declined chemotherapy treatment, 63% of the patients with CD73High and PD-L1 T...
Invivoscribe Announces Updated Reimbursement for the LeukoStrat CDx FLT3 Mutation Assay to Select Newly Diagnosed FLT3-ITD Positive AML Patients Eligible for VANFLYTA in Japan
SAN DIEGO, May 26, 2023 /PRNewswire/ -- Invivoscribe is pleased to announce that their LeukoStrat CDxFLT3 Mutation Assay® has received updated reimbursement byJapan's Ministry of Health, Labor and Welfare (MHLW) to aid in the selection of patients with newly diagnosedFLT3-ITD positive acute my...
Hepagene Therapeutics to Present at the European Association for the Study of the Liver (EASL) Congress 2023
SHANGHAI, May 25, 2023 /PRNewswire/ -- Hepagene Therapeutics, Inc., a clinical stage biopharmaceutical company focusing on developing novel therapies for patients with chronic liver diseases, today announced that Hepagene will present two posters highlighting the preclinical and clinical developm...
HanAll Biopharma and Daewoong Pharmaceutical Enter into Co-Development Agreement with NurrOn Pharmaceuticals to Develop Therapy for Parkinson's Disease
* HanAll Biopharma and Daewoong Pharmaceutical will co-develop NurrOn Pharmaceuticals' novel investigational compounds, including its lead asset ATH-399A, targeting Nurr1 for the treatment of Parkinson's disease (PD), as well as other neurodegenerative disorders * Phase 1 clinical trial is ex...
Telix to Host Briefing with U.S. Leadership Team and Key Opinion Leaders to Highlight Innovation in Urology
June 21, 2023, at The Yale Club, New York City The Company will showcase its urologic pipeline, development of novel therapeutics, imaging agents and technologies that aim to harness the power of targeted radiation at every step of the patient journey MELBOURNE, Australia and INDIANAPOLIS, May 2...
AffaMed Therapeutics Announces Partner Allgenesis Reports Encouraging Preliminary Safety and Efficacy Data from the AG-73305 Phase 2a Trial for the Treatment of Diabetic Macular Edema
* AG-73305 was found to be safe and tolerable with no severe adverse effects (SAEs) after a single intravitreal injection of 0.5 mg and 1 mg in DME patients. * AG-73305 showed median improvements in Best Corrected Visual Accuity (BCVA) of 8.5 ETDRS letters with median Central Subfield Thickness...
111 to Announce First Quarter 2023 Unaudited Financial Results on June 15, 2023 - Conference Call to Follow
SHANGHAI, May 25, 2023 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it will report its unaudited financial results ...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 290 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 289 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00