Pharmaceuticals
Canton Biologics Completes Over 300 Million Yuan Series C Financing, Led by SDIC Venture Capital
GUANGZHOU, China, Sept. 18, 2023 /PRNewswire/ -- On September 13th, Canton Biologics Co., Ltd. announced that it had recently completed a Series C financing round exceeding300 million yuan. This round of financing was led by SDIC Venture Capital, with participation from Guangdong Technology Finan...
Biosyngen received the Asia Pacific Cell & Gene Therapy Excellence Awards (APCGTEA) 2023
SINGAPORE, Sept. 18, 2023 /PRNewswire/ -- On September 14, 2023, Biosyngen Pte Ltd (hereinafter referred to as "Biosyngen") was the recipient of the prestigious "Most Promising Cell Therapy Pipeline in APAC" award at the 7th Annual Cell & Gene Therapy World Asia 2023, amidst a pool of internation...
Samsung Biologics announces expanded strategic agreement with Bristol Myers Squibb to manufacture an antibody cancer drug substance
INCHEON, South Korea, Sept. 17, 2023 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS) announced today a new agreement with Bristol Myers Squibb (NYSE: BMY) for large-scale manufacturing of a Bristol Myers Squibb commercial antibody cancer drug substance. Bristol Myers Squibb and Samsung Biolog...
New drug class prevents key ageing mechanism in organ transplants
ATHENS, Greece, Sept. 18, 2023 /PRNewswire/ -- A novel study has shown that Senolytics, a new class of drugs, have the potential to prevent the transfer of senescence, a key mechanism of ageing, in recipients of older donor organs. The pioneering research, presented today at the European Societ...
YS Biopharma Granted Patent in the U.S. for Immunotherapy Targeting Chronic Hepatitis B Virus Infection
GAITHERSBURG, Md., Sept. 15, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious dise...
Neoss® Group has launched a new wireless intraoral scanner, NeoScan™ 2000
ZURICH, Sept. 15, 2023 /PRNewswire/ -- Neoss Group, a leading innovator in
dental implant solutions, is proud to announce the fast and easy-to-use
wireless intraoral scanner, NeoScan™ 2000. This follows the success of last
year's launch of the NeoScan™ 1000.
Green Leadership Award Presented to Chugai Pharma Taiwan at the Asia Responsible Enterprise Awards 2023
SINGAPORE, Sept. 14, 2023 /PRNewswire/ -- The Asia Responsible Enterprise Awards (AREA) 2023 is pleased to honor 30 outstanding business leaders and enterprises for championing sustainable and responsible business practices. Presented by leading regional NGO Enterprise Asia, the AREA is the mo...
MedAlliance Announces Enrollment of over 1,660 Patients in Landmark SELUTION DeNovo Study
GENEVA, Sept. 15, 2023 /PRNewswire/ -- MedAlliance has announced enrollment of over 1,660 patients in its ground-breaking SELUTION DeNovo coronary randomized study. Recruitment is now half way towards a planned 3,326 patients. SELUTION DeNovo compares the treatment strategy using a novel sirolimu...
Cure Genetics and Frametact Reach a $60 Million Collaboration and Licensing Agreement for the Development of Gene Therapy for Familial Neurological Diseases
SUZHOU, China and HONG KONG, Sept. 14, 2023 /PRNewswire/ -- Cure Genetics and Frametact Limited, a biotech company specializing in researching and developing treatments for neurological diseases, jointly announce the signing of a collaborative development and licensing agreement. The partnership ...
Oscotec/ADEL Announce FDA Clearance of IND Application of ADEL-Y01 for the Treatment of Alzheimer's Disease
PANGYO, South Korea, Sept. 14, 2023 /PRNewswire/ -- Oscotec Inc. and ADEL Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application of ADEL-Y01 for the treatment of Alzheimer's disease (AD). Oscotec and ADEL are jointly developing ...
Porton Advanced and BRL Medicine Build Strategic Partnership to Accelerate Commercialization of Gene and Cell Therapies
SUZHOU, China, Sept. 14, 2023 /PRNewswire/ -- On September 13th, 2023, Porton Advanced Solutions (Porton Advanced) announced a strategic partnership with BRL Medicine Inc. (BRL Medicine). This collaboration will enhance the cell and gene therapy pipelines from BRL Medicine from clinical trials to...
Foundry Mixer Healthcare, Indonesian Minister of Health Budi G. Sadikin: "Together We Leapfrog Indonesia's Healthcare Transformation"
Indonesian Minister of Health, Budi Gunadi Sadikin, emphasized that investing in the Health Technology and Biotechnology sector serves not only business prospects but also strengthens Indonesia's health resilience and adds value to healthcare. JAKARTA, Indonesia, Sept. 14, 2023 /PRNewswire/ -- F...
Dizal Announces China CDE Acceptance of New Drug Application for Golidocitinib for Relapsed or Refractory PTCL
SHANGHAI, Sept. 14, 2023 /PRNewswire/ -- Dizal today announced that the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA) has accepted the New Drug Application (NDA) for golidocitinib for the treatment of relapsed or refractory peripheral T-cell lymphom...
Asieris' New Drug APL-1401 Completes First Administration in Patient with Moderately to Severely Active UC
SHANGHAI, Sept. 14, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that its oral drug APL-1401 for the tr...
Nona Biosciences Expands Antibody Discovery Collaboration with BeiGene
CAMBRIDGE, Mass., Sept. 14, 2023 /PRNewswire/ -- Nona Biosciences, a wholly-owned subsidiary of HBM Holdings Limited, committed to cutting edge antibody technology innovation and provider of integrated antibody discovery and development solutions from "Idea to IND" (I to I™), announced an agreeme...
Innovative Collaboration: Buzzreach and Oncoshot Spearhead Clinical Trial Advancements in Japan
SINGAPORE, Sept. 14, 2023 /PRNewswire/ -- Buzzreach and Oncoshot, two prominent entities in the clinical trials domain, have announced an impactful collaboration to accelerate clinical trials inJapan. The former specialises in clinical trial site recruitment and retention and project managemen...
Exelixis and Insilico Medicine Enter into Exclusive Global License Agreement for ISM3091, a Potentially Best-in-Class USP1 Inhibitor
— ISM3091 is a highly selective, orally bioavailable small molecule inhibitor of USP1 identified through Insilico Medicine's artificial intelligence (AI) platform, with potent activity in BRCA-mutated tumor models — — In May 2023, the U.S. Food and Drug Administration (FDA) cleared Insilico's I...
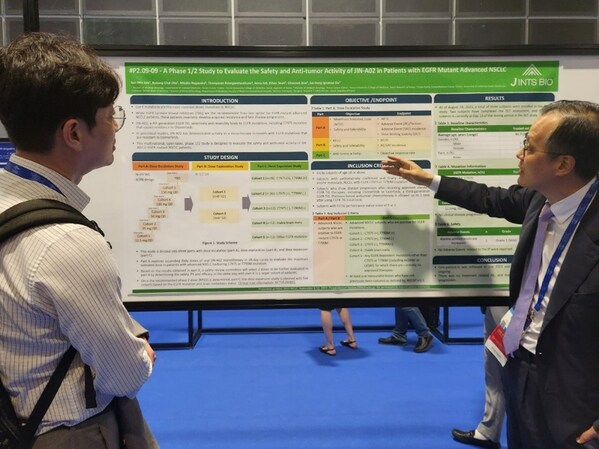
J INTS BIO, WCLC 2023 - Presentation of Phase 1/2 study of 'JIN-A02', a Novel Oral 4th Generation EGFR TKI
SEOUL, South Korea, Sept. 13, 2023 /PRNewswire/ -- J INTS BIO announced that Phase 1/2 study of its novel, orally administered 4th generation EGFR-TKI 'JIN-A02' was presented at the 2023 IASLC World Conference on Lung Cancer held inSingapore from 9th to 12th September, during the official session...
Zuberitamab (Anruixi®), the first domestically developed anti-CD20 antibody as a Class I innovative drug from BioRay Pharmaceutical, has been approved for marketing in China
SHANGHAI, Sept. 13, 2023 /PRNewswire/ -- Recently, BioRay Pharmaceutical Co., Ltd. (hereinafter referred to as "Bioray") announced that its independently developed Class I innovative therapeutic biological product, Zuberitamab Injection (trade name: Anruixi®), indicated for the treatment of CD20...
Caliway Announces CBL-514 Phase 2 Study Topline Results for Dercum's Disease, Showing 64.5% of Lipomas with Complete Clearance or Dimensions Reduction of Over 50% and Reduced Pain by 4.7
- CBL-0201DD Phase 2 study met the primary and all secondary endpoints. - 64.5% of painful lipomas showed complete clearance or dimensions reduction of more than 50% after CBL-514 treatments. - 54.8% of painful lipomas showed complete clearance or dimension reduction of more th...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 290 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 289 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00