Pharmaceuticals
Medicilon has appointed Dr. Qingcong Lin as President of Medicilon USA Corp., further deepening the global strategic layout
BOSTON, March 11, 2024 /PRNewswire/ -- Medicilon, a one-stop pharmaceutical preclinical research and developmentCRO can simultaneously meet Chinese and American drug discovery and development needs (including GLP standards) promptly and efficiently with fast initiation times.Over the past 20 year...
Ascletis Announces Poster Presentation of Phase II Study Final Results of FASN Inhibitor ASC40 for Treatment of Acne at 2024 AAD Annual Meeting
HANGZHOU and SHAOXING, China, March 10, 2024 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX: 1672, "Ascletis") today announces the poster presentation of Phase II study final results of ASC40, a first-in-class fatty acid synthase (FASN) inhibitor for treatment of acne, at the 2024 American Academy of...
Regor Initiates Phase 2 Study of Oral Once-daily GLP-1 Agonist RGT-075 for the Treatment of Obesity
CAMBRIDGE, Mass., March 8, 2024 /PRNewswire/ -- Regor Therapeutics Group ("Regor"), a clinical-stage global biopharmaceutical company powered by a cutting-edge drug discovery engine and differentiated clinical development pipeline, today announced that its Phase 2 trial of the highly selective or...
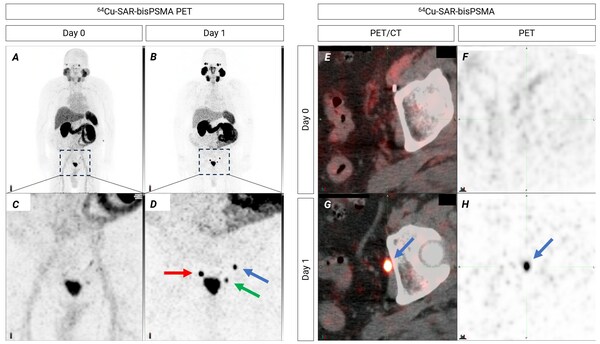
Additional COBRA results: SAR-bisPSMA detects lesions in the 2-millimetre range
Highlights * Clarity recently reported that in its diagnostic Phase 1/2 trial, COBRA, 64 Cu-SAR-bisPSMA was found to be safe and highly effective in detecting prostate cancer (PC) lesions in patients with biochemical recurrence (BCR). * In trial participants where standard of care (SOC) imagin...
Mabwell to Present the Clinical Data of 9MW2821 in Cervical Cancer as Focused Plenary Oral Presentation at 2024 Society of Gynecologic Oncology Annual Meeting on Women's Cancer
SHANGHAI, March 8, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present the clinical study results on the efficacy and safety of the novel Nectin-4-targeting ADC 9MW2821 for patients with recurrent or ...
TransThera Announced Global Phase 3 Clinical Trial for Cholangiocarcinoma Authorized in the European Union and Orphan Drug Designation for Tinengotinib to Treat Biliary Tract Cancer Granted by European Medicines Agency
NANJING, China and GAITHURSBURG, Md., March 8, 2024 /PRNewswire/ -- TransThera, a clinical-stage biopharmaceutical company dedicated to innovating differentiated drugs globally, today announced that the randomized, controlled, global multicenter Phase 3 trial (FIRST-308) of tinengotinib versus ph...
VISEN Announces Acceptance of a Biologics License Application for Lonapegsomatropin in China
SHANGHAI, March 7, 2024 /PRNewswire/ -- VISEN Pharmaceuticals (VISEN), an innovative biopharmaceutical company focused on endocrine diseases, today announced that the Biologics License Application (BLA) for Lonapegsomatropin (TransCon hGH) was accepted by the China National Medical Products Admi...
Chime Biologics Achieves ISO 27001 Certification to Strengthen Information Security in the CDMO Industry
WUHAN, China, March 7, 2024 /PRNewswire/ -- Chime Biologics, a leading global CDMO that enables its partners' success in biologics, today announced that it has obtained ISO/IEC 27001:2022 certification on information security management systems. After passing rigorous reviews on all the items of...
Akeso Announced the First Patient Dosed in Phase III Trial of Cadonilimab(PD-1/CTLA-4) Combined with Chemotherapy versus Tislelizumab Combined with Chemotherapy in First-line Treatment of PD-L1 negative NSCLC
HONG KONG, March 6, 2024 /PRNewswire/ -- Akeso Inc. ("Akeso", 9926. HK) announced the enrollment of the first patient in the registrational Phase III clinical study comparingCadonilimab (PD-1/CTLA-4 bispecific antibody) combined with chemotherapy versus Tislelizumab (PD-1 antibody) combined with ...
AACR 2024 | Ascentage Pharma to Present Three Preclinical Studies at 2024 American Association of Cancer Research Annual Meeting
ROCKVILLE, Md. and SUZHOU, China, March 5, 2024 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancer, age-related diseases, and chronic hepatitis B (CHB), announced today that the latest results from three preclinical stud...
YS Biopharma Announces Board Changes and Strategic Leadership Appointments in Chinese Subsidiaries
GAITHERSBURG, Md., March 5, 2024 /PRNewswire/ -- YS Biopharma Co., Ltd. (Nasdaq: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disea...
WuXi Biologics Achieves Leadership Level in 2023 CDP Climate Change Assessment
SHANGHAI, March 5, 2024 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a leading global Contract Research, Development and Manufacturing Organization (CRDMO), announced it has been recognized for outstanding performance in the 2023 Climate Change Assessment conducted by the global environ...
Formosa Pharmaceuticals and AimMax Therapeutics Receive FDA Approval for Clobetasol Propionate Ophthalmic Suspension 0.05%, for the Treatment of Post-Operative Inflammation and Pain Following Ocular Surgery
TAIPEI, March 4, 2024 /PRNewswire/ -- Taiwan-based Formosa Pharmaceuticals (6838.TWO) and AimMax Therapeutics (United States) announced today that the U.S. Food and Drug Administration (FDA) has approved clobetasol propionate ophthalmic suspension 0.05% (APP13007), for the treatment of post-opera...
ACROBiosystems Launches New Initiative to Support Cell and Gene Manufacturers
NEWARK, Calif., March 4, 2024 /PRNewswire/ -- ACROBiosystems, one of the leading providers of innovative tools and solutions used from discovery to the clinic, has announced a new corporate initiative to supportex vivo cell manufacturing and manufacturers of innovative cell and gene therapies. I...
Henlius Forecasts Profit in 2023: Achieving first full year of profitability, and ushering in a new phase of high-quality development
SHANGHAI, March 4, 2024 /PRNewswire/ -- Henlius (2696.HK) released a positive profit forecast. Based on the preliminary assessment of the unaudited consolidated management accounts for the year ended31 December, 2023 (the "Reporting Period") and the information currently available to the Board,it...
Landmark Clinical Approval for YOLT-201 Obtained by the NMPA
SHANGHAI, March 4, 2024 /PRNewswire/ -- YolTech Therapeutics, a biotech company developing in vivo gene editing therapies to treat rare genetic diseases, today announced the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) has officially approved the YOLT-2...
Caliway Announced Orphan Drug Designation Granted to CBL-514 for the Treatment of Dercum's Disease
- CBL-514 is the first and only drug to receive both Orphan Drug Designation and Fast Track designation for Dercum's disease treatment. NEW TAIPEI CITY, March 3, 2024 /PRNewswire/ -- Caliway Biopharmaceuticals (Caliway) announced that the U.S. Food and Drug Administration (FDA) granted Orp...
NMPA Approves the NDA for CARsgen's BCMA CAR-T Therapy Zevorcabtagene Autoleucel for Relapsed or Refractory Multiple Myeloma
SHANGHAI, March 1, 2024 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that today the National Medical Products Administration ("NMPA") ofChi...
111 to Announce Fourth Quarter and Fiscal Year 2023 Unaudited Financial Results- Conference Call to Follow
SHANGHAI, March 1, 2024 /PRNewswire/ -- 111, Inc. (NASDAQ: YI) ("111" or the "Company"), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced that it will report its unaudited financial results...
Jacobio Pharma Receives IND Approval for P53 Y220C Activator JAB-30300 in the U.S.
BEIJING, SHANGHAI and BOSTON, March 1, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company drugging the undruggable targets, today announced it received IND (Investigational New Drug) approval of its self-developed drug JAB-30300 (P53 Y220C activator) from the FDA of ...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 292 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 290 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00