SYDNEY, Nov. 10, 2021 /PRNewswire/ --Clarity Pharmaceuticals (ASX: CU6) ("Clarity" or the "Company"), an Australian-based clinical stage radiopharmaceutical company developing next-generation products to address the growing need in oncology, is pleased to announce that it has completed recruitment for the initial dosimetry phase of its US-based SECuRE clinical trial (NCT04868604)[1] investigating SAR-bisPSMA Targeted Copper Theranostics (TCT) in patients with metastatic castrate-resistant prostate cancer (mCRPC).
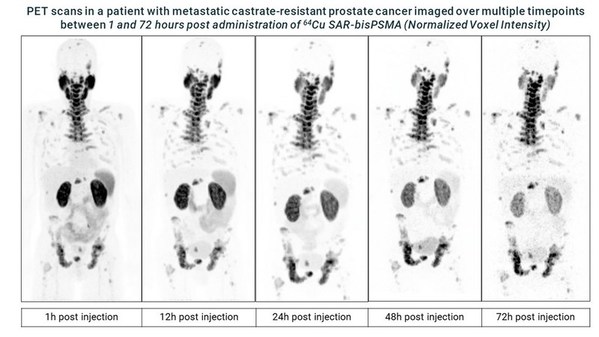
PET scans in a patient with metastatic castrate-resistant prostate cancer imaged over multiple timepoints between 1 and 72 hours post administration of Cu-64 SAR-bisPSMA (Normalized Voxel Intensity)
The SECuRE trial is a Phase I/IIa theranostic trial for identification and treatment of prostate specific membrane antigen (PSMA)-expressing mCRPC using TCT. 64Cu SAR-bisPSMA is used to visualise PSMA expressing lesions and select candidates for subsequent 67Cu SAR-bisPSMA therapy. The initial dosimetry phase utilised 64Cu SAR-bisPSMA to determine biodistribution and dosimetry of the products in humans. The SECuRE trial is a multi-centre, single arm, dose escalation study with a cohort expansion planned for up to 44 patients in the US. The aim of this trial is to determine the safety and efficacy of 67Cu SAR-bisPSMA as a therapy.
Clarity's Executive Chairman, Dr Alan Taylor, commented, "We are very pleased to have quickly and successfully completed the recruitment for the initial dosimetry phase of the SECuRE trial in mCRPC using our optimised next-generation PSMA agent, SAR-bisPSMA, and look forward to progressing through the safety review shortly. We are excited to move quickly to the therapy phase with 67Cu SAR-bisPSMA at all seven clinical sites selected for this trial in the US.
"The PET imaging data acquired in the SECuRE trial to date looks very promising and the images confirm our excellent preclinical results of high tumour targeting and retention whilst seeing washout in other tissues. We are excited with the comparison to the standard of care bone scan (the recommended modality for bone imaging in clinical trials according to the Prostate Cancer Clinical Trials Working Group 3), indicating that 64Cu SAR-bisPSMA is able to visualise bone involvement. This further supports the emerging evidence of increased sensitivity and specificity of PSMA PET tracers for detecting micrometastatic disease compared to conventional imaging. With the recently updated US National Comprehensive Cancer Network Guidelines® now allowing FDA-approved PSMA PET agents to be used as an alternative to conventional imaging, we are really looking forward to progressing this product quickly through clinical trials with the added value of manufacturing, logistics and patient benefits that 64Cu provides.
"Our TCT platform uniquely uses the same chemical entity for both diagnosis and therapy, leading to high accuracy and high precision, and highlights the benefits of generating 64Cu imaging data from 1h to 72h after administration to help determine the suitability of treatment with 67Cu. We strongly believe SAR-bisPSMA will be an important pillar in the next generation of radiopharmaceuticals, with blockbuster potential both diagnostically and therapeutically. The central manufacture, logistical and treatment advantages of TCT associated with using the isotope pairing of 64Cu and 67Cu in large patient populations such as prostate cancer will provide significant benefits to both patients and clinicians in comparison to current products in the market."
Dr Luke Nordquist, CEO, Urologic Medical Oncologist at the Urology Cancer Center and GU Research Network in Omaha, Nebraska, who treated patients in the initial dosimetry phase of the trial, commented, "I am very impressed with the data from the initial dosimetry phase and we look forward to progressing the SECuRE trial into the therapy phase and further validating the benefits of the 64Cu SAR-bisPSMA and 67Cu SAR-bisPSMA products for both clinicians and patients. Current standard of care SPECT imaging agents don't have the resolution of the new PET agents, however the new PET agents rely on radionuclides with very short half-lives such as 18F and 68Ga, which limits their availability and utilisation. Having access to centrally manufactured PET imaging products with a more suitable half-life, such as 64Cu, will significantly improve patient care and address the current backlog of patients waiting for critical imaging scans. Importantly, the potential for improved prostate cancer diagnosis and treatment will have significant benefits for prostate cancer patients."
Dr Taylor said: "The future of radiopharmaceuticals is here, where patient care is not dictated by the limited half-life of the isotope, and instead focuses on what is important for the patient, clinician and treating staff – safety, efficacy, access and flexibility. The further expansion of radiopharmaceuticals into more indications, with greater utilisation from a broad spectrum of clinicians, will be dependent upon centralised, large volume and simple supply logistics of isotopes and ready-to-use radiopharmaceuticals whilst focusing on long-term environmental impacts of the supply chain. Clarity's ability to centrally manufacture and broadly distribute large volumes of copper-based products, without long lived radioactive waste products or dependance on nuclear reactors, will be a significant factor in addressing large markets in a sustainable manner. To that end, we are very excited to be quickly advancing our TCT platform and numerous TCT products through clinical trials to generate compelling clinical data and move closer to achieving our ultimate goal of developing better treatments for children and adults with cancer."
This announcement has been authorised for release by the Executive Chairman.
About Prostate Cancer
Prostate cancer is the second most common cancer diagnosed in men globally and the fifth leading cause of cancer death worldwide[2]. In 2021, the National Cancer Institute estimated 248,530 new cases of prostate cancer in the US and around 34,130 deaths from the disease[3]. Annually, there are around ~34,000 men in the US who are diagnosed with mCRCP[4], ~90% of whom have tumours which express PSMA[5].
References
1. ClinicalTrials.gov Identifier: NCT04868604 https://clinicaltrials.gov/ct2/show/NCT04868604
2. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660
3. American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate
4. American Cancer Society, Cancer Statistics Center, https://cancerstatisticscenter.cancer.org/?_ga=2.79808020.284532473.1620009137-1916069442.1615761164#!/cancer-site/Prostate
5. D. A. Silver, I. Pellicer, W. R. Fair, W. D. Heston and C. Cordon-Cardo 1997. "Prostate-specific membrane antigen expression in normal and malignant human tissues." Clinical Cancer Research. vol. 3, 81-85, January 1997
For more information, please contact:
Dr Alan Taylor |
Simon Hinsley |
Executive Chairman |
Investor/Media Relations |
+61 401 809 653 |
About Clarity Pharmaceuticals
Clarity is a clinical stage radiopharmaceutical company focused on the treatment of serious disease. The Company is a leader in innovative radiopharmaceuticals, developing targeted copper theranostics based on its SAR Technology Platform for the treatment of cancer in children and adults.
www.claritypharmaceuticals.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/recruitment-for-the-dosimetry-phase-of-claritys-cu-64cu-67-sar-bispsma-theranostic-prostate-cancer-trial-completed-301420839.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/recruitment-for-the-dosimetry-phase-of-claritys-cu-64cu-67-sar-bispsma-theranostic-prostate-cancer-trial-completed-301420839.html