Medical/Pharmaceuticals
CSL Vifor and Travere Therapeutics announce sparsentan receives positive CHMP opinion for the treatment of IgA nephropathy
Committee for Medicinal Products for Human Use (CHMP) recommends approval of the conditional marketing authorization (CMA) for sparsentan for the treatment of IgA nephropathy (IgAN) inEurope Positive CHMP opinion is based on pivotal phase-III PROTECT study results European Commission decision is...
Immunofoco Announces the Dual Approval of IND Applications by the U.S. FDA and China CDE for the First EpCAM CAR-T Targeted at Advanced Solid Tumors
- The first EpCAM targeted CAR-T product obtained US/CN IND approval. - Acceptable safety profiles and preliminary efficacy were observed in Investigator-Initiated Trial (IIT) clinical studies of IMC001. SHANGHAI, Feb. 22, 2024 /PRNewswire/ -- Immunofoco, a company dedicated to developing cell ...
Jolly Good Revolutionizes Healthcare with Immersive Service for Apple Vision Pro, Premiering at SXSW 2024
A Milestone Entry into Spatial Computing Healthcare BROOKLINE, Mass., Feb. 22, 2024 /PRNewswire/ -- Jolly Good US Inc. proudly presents "JOLLYGOOD+ for Vision Pro," a groundbreaking immersive medical service harnessing the power of spatial computing through Apple Vision Pro technology. This inno...
CATUG and Crystal Bio Establish Strategic Partnership, Launching "CATUG-Crystal" Joint Lab Dedicated to Advanced Nucleic Acid Analytical Services
CAMBRIDGE, Mass. and CRANBURY, N.J., Feb. 22, 2024 /PRNewswire/ -- CATUG Inc. (CATUG) andCrystal Bio, a member of Crystal Pharmatech, announced today a long-term strategic partnership to provide advanced nucleic acid-based drug analytical services. CATUG, a distinguished global entity specializin...
Kakao Brain Debuts 'KARA-CXR', a Web-Based Radiology Report Service Powered by Large-Scale AI
"AI Assistant for Radiologists" * Kakao Brain introduced last month a web-based radiology report Research Use Only (RUO) service powered by its large-scale artificial intelligence (AI), "KARA-CXR." * Kakao Brain plans to expand the interactive functions supporting various imaging medical exa...
Telix 2023 Full Year Results: Inaugural Profit Achieved, Strong Revenue Growth Underpins Investment in Late-stage Pipeline
MELBOURNE, Australia, Feb. 22, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today announces its results for the financial year ended31 December 2023. All figures are in AUD$ unless otherwise stated.[1] 2023 highlights * Total Group revenue of $502.5M, an...
Everest Medicines' Partner Pfizer Announces European Commission Approves VELSIPITY® for Patients with Moderately to Severely Active Ulcerative Colitis
--VELSIPITY is the first and only oral advanced ulcerative colitis therapy approved for use in patients 16 years of age or older in the EU-- SHANGHAI, Feb. 22, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company")'s partner Pfizer Inc. (NYSE: PFE) announced that the ...
VOCIC Presents its Latest Products at Arab Health 2024 in Dubai
CITY OF INDUSTRY, Calif., Feb. 21, 2024 /PRNewswire/ -- The VOCIC team showcased its latest home medical care and senior care products at Arab Health 2024 in Dubai, held from January 29th to February 1st. The exhibition provided a crucial chance to present VOCIC's innovative healthcare solutions t...
Mabwell Publishes the Phase III Study Results on Its Denosumab Biosimilar (MW032) in the journal JAMA Oncology
SHANGHAI, Feb. 21, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, recently published the phase III study results of denosumab biosimilar (MW032) online in the international top journal of JAMA Oncology. This is the first recorded tria...
Zylox-Tonbridge Receives Marketing Approvals for Multiple Products in the UAE
HANGZHOU, China, Feb. 20, 2024 /PRNewswire/ -- Zylox-Tonbridge (2190.HK), a medical device company specializing in peripheral and neurovascular interventions, proudly announces the recent marketing approvals granted by the Ministry of Health and Prevention in theUnited Arab Emirates for five of i...
Astonishing brain tumour research wins the BIAL Award in Biomedicine 2023 worth 300,000 Euro
PORTO, Portugal, Feb. 21, 2024 /PRNewswire/ -- A team of researchers from
Germany, the USA, the UK, and Norway won the third edition of the BIAL Award in
Biomedicine
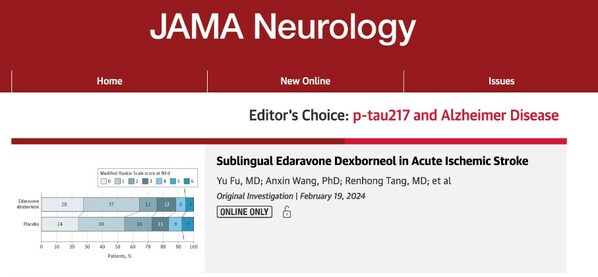
Findings of a Phase III Clinical Study of Sanbexin® Sublingual Tablets Published in JAMA
NANJING, China, Feb. 21, 2024 /PRNewswire/ -- On February 19, 2024, the Journal of American Medical Association•Neurology (JAMA NEUROLOGY, IF: 29.0) published online the key findings of a phase III clinical study (NCT04950920) (the "TASTE-SL Study") investigating Sanbexin® (a combination of edara...
Ono Enters into a Research Collaboration Agreement with InveniAI to Identify Novel Therapeutic Targets
OSAKA, Japan, Feb. 20, 2024 /PRNewswire/ -- Ono Pharmaceutical Co., Ltd. ( Osaka, Japan; President and CEO: Gyo Sagara; "Ono") today announced that it has entered into a research collaboration agreement with InveniAI® LLC (Guilford, Connecticut, USA; President and CEO: Krishnan Nandabalan; "Inveni...
Everest Medicines' Licensing Partner Venatorx Pharmaceuticals Announces Publication of Positive Results from Cefepime-Taniborbactam's Phase 3 CERTAIN-1 Study in New England Journal of Medicine
--Cefepime-taniborbactam was superior to meropenem for the composite efficacy endpoint with composite efficacy sustained at late follow-up visit-- SHANGHAI, Feb. 21, 2024 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK)'s licensing partner, Venatorx Pharmaceuticals announced that The New England ...
Datasea Establishes Joint Venture Company to Further Penetrate the Estimated Approximately 527.8 billion US Dollars 5G Market in China
New Entity to be Focused on Increasing Scale of Datasea's 5G Services Utilizing Artificial Intelligence, which is Expected to Drive Significant Revenue in 2024 BEIJING, Feb. 20, 2024 /PRNewswire/ -- Datasea Inc. (NASDAQ: DTSS) ("Datasea" or the "Company"), aNevada incorporated digital technology ...
Hox Therapeutics and Vernalis announce a drug discovery collaboration in oncology
CHENGDU, China, Feb. 20, 2024 /PRNewswire/ -- Hox Therapeutics Ltd ("Hox") a private biotechnology company developing highly targeted cancer therapies and Vernalis (R&D) Ltd ("Vernalis"), a fully owned subsidiary of HitGen Inc., are pleased to announce a collaboration to identify inhibitors again...
Mabwell Receives IND Approval from FDA for Novel B7-H3 ADC 7MW3711
SHANGHAI, Feb. 20, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that its clinical trial application of B7-H3 targeting ADC (R&D code: 7MW3711) for advanced malignant solid tumor was approved by the U.S. Food and Drug Admi...
FDA Grants Orphan Drug Designation to 9MW3011
SHANGHAI, Feb. 20, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative biopharmaceutical company with entire industry chain, announced that FDA has granted Orphan Drug Designation (ODD) to 9MW3011 (R&D code in the US: MWTX-003/DISC-3405) for the treatment of patients with polycythemia vera (P...
WuXi Advanced Therapies Receives FDA Approval to Manufacture Iovance's AMTAGVI™ (lifileucel) for Advanced Melanoma
AMTAGVI is the first and only one-time, individualized T cell therapy to receive U.S. FDA approval fora solid tumor cancer. PHILADELPHIA, Feb. 20, 2024 /PRNewswire/ -- WuXi Advanced Therapies (WuXi ATU), a wholly owned subsidiary of WuXi AppTec, today announced that the U.S. Food and Drug Admini...
Kangpu Biopharmaceuticals to Present Preclinical Efficacy Data of KPG-818 in Crohn's Disease at the 19th Congress of ECCO (IND Application for Phase II in Progress)
HEFEI, China, Feb. 20, 2024 /PRNewswire/ -- Kangpu Biopharmaceuticals, a clinical-stage company dedicated to the discovery and development of novel therapeutics for the treatment of cancer, autoimmune diseases, and inflammation, through targeted protein degradation technology, today announced th...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
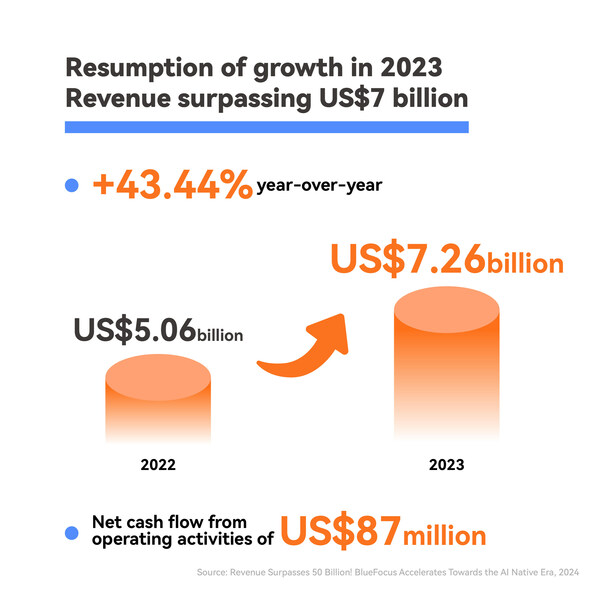
2024-04-19 13:25Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 273 media titles]
2024-04-24 17:09