Pharmaceuticals
GC Biopharma to organize "Donate Blood with Love", a healthy way of giving back to the community
With the participation of all affiliated group entities YONGIN, South Korea, Aug. 30, 2023 /PRNewswire/ --All executives and employees of GC Biopharma, a South Korean biopharmaceutical company, rolled up their sleeves to practice ESG and social value. On Aug. 30th, the company held the "Donate...
Asieris Obtained IND Approval from NMPA for APL-1401, a Drug for the Treatment of Moderately-to-Severely Active Ulcerative Colitis
SHANGHAI, Aug. 30, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that the National Medical Products Adm...
InxMed Receives Approval to Initiate Phase I Clinical Trial in China for OMTX705, a First-in-Class FAP-Targeting ADC
NANJING, China, Aug. 29, 2023 /PRNewswire/ -- InxMed, a clinical-stage biotechnology company dedicates on developing innovative therapies targeting drug resistance for hard-to-treat solid tumors,announced today the first-in-class ADC OMTX705 has obtained the IND (Investigational New Drug) approv...
Fosun Pharma Announces 2023 Interim Results: Continues to Promote Innovation Transformation and Optimizes Product Structure
SHANGHAI, Aug. 29, 2023 /PRNewswire/ -- On August 29, 2023, Shanghai Fosun Pharmaceutical Co., Ltd.* (Stock Code: 600196.SH; 02196.HK) announced its 2023 Interim Results for the first half of 2023. (the "Reporting Period"). During the Reporting Period, Fosun Pharma achieved revenue ofRMB21.395 bi...
Akeso Announced 2023 Interim Results: First Profit, Growing Sales of PD-1/CTLA-4 Bispecific Antibody and Priority Review of PD-1/VEGF
—During the reporting period, Akeso's revenue was RMB3,676.9 million, an increase of 2,154.4% from 2022H1; Akeso recorded a profit ofRMB2,489.5 million. —RMB2,915.2 million was recognized by Akeso as license fee income during the reporting period due to the receipt of an upfront payment equivalen...
Cerecin to Present New Infantile Spasms Data at the 35th International Epilepsy Congress, Dublin 2023
* Data from the pilot study in infantile spasms showed positive outcomes. * The results will be presented by Cerecin's Chief Medical Officer, Dr. Marc Cantillon on Sunday 2nd September at 2pm (GMT+1). * The Cerecin team will be accompanied by PharmaVentures, who will be supporting Cerecin's ...
Akeso Announced Completion of Patient Enrollment in Phase 3 Trial of Ivonescimab (PD-1/VEGF) versus Pembrolizumab in First-line PD-L1 Positive Advanced NSCLC
HONG KONG, Aug. 29, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", 9926. HK) announced completion of patient enrollment in a head-to-head study of ivonescimab (AK112, PD-1/VEGF bispecific antibody) compared with pembrolizumab as first-line treatment for patients with PD-L1 positive (PD-L1 TPS≥1%) loca...
ProfoundBio to Participate in the 21st Annual Morgan Stanley Conference
SEATTLE, Aug. 28, 2023 /PRNewswire/ -- ProfoundBio, a clinical-stage biotechnology company focused on the development of novel antibody-drug conjugate therapeutics for cancer, announced that management will be participating in one-on-one meetings at the Morgan Stanley 21st Annual Global Healthca...
Harbour BioMed Announces 2023 Interim Results
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 28, 2023 /PRNewswire/ -- Harbour BioMed ("HBM", or the "Company"; HKEX: 02142), a global biopharmaceutical company committed to the discovery, development, and commercialization of novel antibody therapeutics focusing on oncology an...
Harbour BioMed Announces US IND Clearance of Its First ADC Program HBM9033 in Solid Tumors
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SUZHOU, China, Aug. 28, 2023 /PRNewswire/ -- Harbour BioMed (the"Company", HKEX: 02142) announced that the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application to commence clinical trials of its first antib...
Specialised Therapeutics acquires commercialisation rights to new oral MND therapy
.. ST to partner with Dutch biotech company Treeway BV .. First CNS therapy in ST therapeutic portfolio SINGAPORE, Aug. 27, 2023 /PRNewswire/ -- Independent biopharmaceutical company Specialised Therapeutics Asia Pte Ltd (ST) will partner withNetherlands based biotechnology company Treeway BV to...
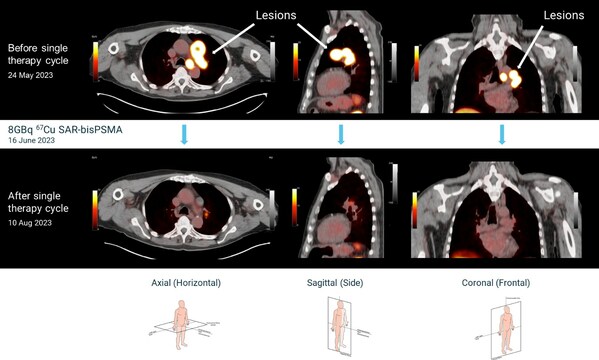
First participant treated at the highest dose level in Clarity's theranostic prostate cancer trial
Highlights * First participant of cohort 3 in the theranostic SECuRE trial investigating 64Cu/67Cu SAR-bisPSMA in metastatic castrate-resistant prostate cancer (mCRPC) has been treated at the highest dose level of 12GBq. * Cohort 2 was recently completed in 3 participants who received therapy...
Akeso Announced that Ivonescimab Granted Priority Review of NDA by China NMPA
HONG KONG, Aug. 25, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", the "Company"; 9926.HK), a commercial-stage biopharmaceutical company focused on developing and commercializing first-in-class and best-in-class innovative medicines globally, announced today that the National Center for Drug Evaluatio...
Tigermed Reports 2023 Interim Results
HANGZHOU, China, Aug. 25, 2023 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. ("Tigermed" or the "company") (Stock code: 300347.SZ / 3347.HK), a leading provider of clinical research solutions across full lifecycle of global biopharmaceutical and medical device products, announced its int...
GC Biopharma to Produce Cholera Vaccines Jointly with Eubiologics
YONGIN, South Korea, Aug. 25, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a South Korean biopharmaceutical company, announced today that it has signed a MOU at its headquarters in Yongin,South Korea with Eubiologics for a co-production of Euvichol, an oral cholera vaccine. Under the MOU, bo...
Akeso Announced NDA Acceptance of IL-12/lL-23 Monoclonal Antibody Ebdarokimab for Moderate-to-severe Plaque Psoriasis by China NMPA
HONG KONG, Aug. 24, 2023 /PRNewswire/ -- Akeso Inc. ("Akeso", the "Company"; 9926.HK), a commercial-stage biopharmaceutical company focused on developing and commercializing first-in-class and best-in-class innovative medicines globally, announced today that the New Drug Application (NDA) for its...
111, Inc. Announces Second Quarter 2023 Unaudited Financial Results
SHANGHAI, Aug. 24, 2023 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced its unaudited financial results for the second quar...
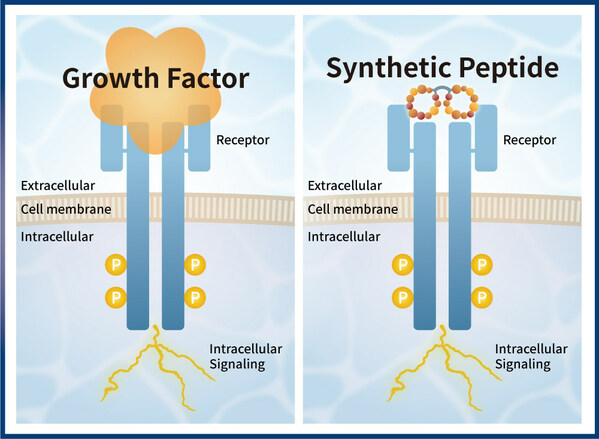
PeptiGrowth Inc. and Orizuru Therapeutics, Inc. Enter into Joint Development of Novel Synthetic Peptide Based Growth Factor
TOKYO, Aug. 23, 2023 /PRNewswire/ -- PeptiGrowth Inc (PeptiGrowth), a Japanese biotechnology company focusing on the development of synthetic peptide based growth factors and Orizuru Therapeutics, Inc(OZTx), a Japanese biopharmaceutical company focusing on the research and development of iPSC-de...
Samsung Biologics appoints new leadership to oversee quality and regulatory affairs
- Distinguished industry leaders with extensive track records in quality assurance and regulatory affairs join Samsung Biologics to support the company's quality-driven business operation. INCHEON, South Korea, Aug. 23, 2023 /PRNewswire/ -- Samsung Biologics (KRX: 207940.KS), a global contract d...
Maypharm Launches the world first Adipose Stem Cell Exosome hair filler, HAIRNA Exosome Hair Fill
SEOUL, South Korea and NEW YORK, Aug. 23, 2023 /PRNewswire/ -- Maypharm
Launches the world first Adipose Stem Cell Exosome hair filler, HAIRNA Exosome
Hair Fill. HAIRNA Exosome Hair Fill is developed with Maypharm's exclusive
technology for scalp rejuvenation and hair growth.
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 272 media titles]
2024-04-24 17:09