Pharmaceuticals
FDA Grants Orphan Drug Designation to Cevidoplenib for ITP
PANGYO, South Korea, March 21, 2024 /PRNewswire/ -- Oscotec Inc. has secured orphan drug designation (ODD) from the U.S. Food and Drug Administration (FDA) for its SYK inhibitor, Cevidoplenib, to treat immune thrombocytopenia (ITP). Oscotec has successfully completed phase 2 study in patients wit...
HanAll Biopharma Reports Full-Year 2023 Financial Results and Provides Business Update
* Full-year 2023 net revenue reaches 135 billion KRW, driven by strong sales growth. * R&D momentum persisted with promising developments in anti-FcRn antibodies HL161ANS and batoclimab, positioning them as potentially best-in-class treatments for lgG-mediated autoimmune diseases. * Anticip...
Biosion to Present Three Posters at the 2024 AACR Meeting
NEWARK, Del. and NANJING, China, March 21, 2024 /PRNewswire/ -- Biosion, a global, clinical-stage biotechnology company, today announced upcoming presentations of pre-clinical data for its oncology pipeline assets, including BSI-111, an anti-CD16a monoclonal antibody, BSI-730, a HER2/PD-L1 bispec...
111, Inc. Announces Fourth Quarter and Fiscal Year 2023 Financial Results
SHANGHAI, March 21, 2024 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a leading tech-enabled healthcare platform company committed to digitally connecting patients with medicine and healthcare services inChina, today announced its unaudited financial results for the fourth qua...
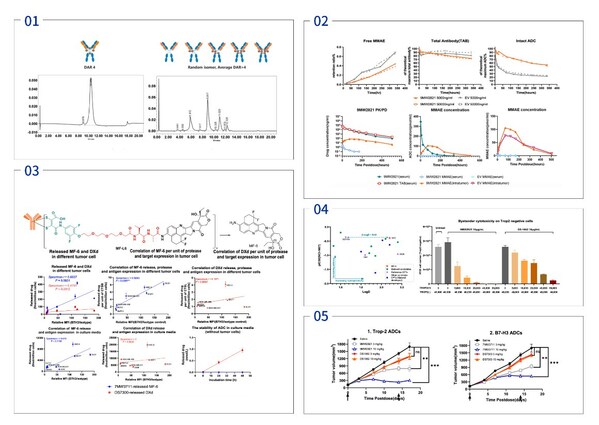
Mabwell Presented IDDC™ Platform Technology and ADC Drug Development Achievements at the 14th World ADC London
SHANGHAI, March 21, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, published "Mtoxin™ Payload Applied in IDDC™ ADC Platform Significant Increases Therapeutic Index and Overcome MultiDrug Resistance in Various Tumor" as poster ...
China Jo-Jo Drugstores Regains Compliance with Nasdaq Minimum Bid Price Requirement
HANGZHOU, China, March 19, 2024 /PRNewswire/ -- China Jo-Jo Drugstores, Inc. (NASDAQ: CJJD) ("Jo-Jo Drugstores" or the "Company"), a leading online and offline retailer, wholesale distributor of pharmaceutical and other healthcare products and healthcare provider inChina, today announced that it ...
SGO 2024 | The First Published Clinical Data of Nectin-4-Targeting ADC Developed by Mabwell in Cervical Cancer Demonstrates Its Outstanding Therapeutic Potential
SHANGHAI, March 19, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovative-driven biopharmaceutical company with entire industry chain, presented the clinical study data of the 9MW2821 for patients with cervical cancer as focused plenary oral presentation at the Society of Gynecologic Oncology ...
Breakthrough in Treatment of Cervical High-Grade Squamous Intraepithelial Lesion: Release of Positive Results from Multicenter Phase III Global Clinical Study
* The world's first non-surgical cervical HSIL treatment validated by an international Phase III clinical study with proven efficacy; * The response rate increased by 89.4% compared to the placebo control group, with a low incidence of adverse events; * The China new drug application submiss...
WuXi AppTec Revenue Surpassed RMB40 Billion in 2023, and Adjusted Non-IFRS Net Profit Exceeded RMB10 Billion for the First Time
* Revenue Up 2.5% Year-over-Year to RMB40,341 Million; Excluding COVID-19 Commercial Projects, Up 25.6% * Net Profit Attributable to the Owners of the Company[1] Increased 9.0% to RMB9,607 Million; Diluted Earnings per Share (EPS) Increased 14.9% to RMB3.24 * Adjusted Non-IFRS Net Profit Att...
Positive results from Phase II clinical trial (CGZ203 study) of Chiglitazar monotherapy for non-alcoholic steatohepatitis
SHENZHEN, China, March 18, 2024 /PRNewswire/ -- On March 18 2024, Shenzhen chipscreen biosciences Co., Ltd. (hereinafter referred to as "chipscreen biosciences", stock code: 688321. SH) announced that the Phase II clinical trial (CGZ203 study) of chiglitazar monotherapy for non-alcoholic steatoh...
KeChow Pharma Announces NMPA Approval of Tunlametinib (HL-085) as the First Targeted Therapy for Patients with NRAS Mutated Advanced Melanoma and Previously Treated with PD-1/PD-L1
-- Tunlametinib is poised to be a potential best-in-class MEK inhibitor based on positive data from the pivotal Phase 2 study SHANGHAI, March 18, 2024 /PRNewswire/ -- KeChow Pharma, a commercial-stage pharmaceutical company focused on developing and commercializing differentiated small molecule ...
ImmVira's oncolytic product MVR-T3011 IT Intratumoral Injection Receives FDA Fast Track Designation for HNSCC Treatment
SHENZHEN, China, March 15, 2024 /PRNewswire/ -- ImmVira has recently announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for oncolytic virus product MVR-T3011 IT (intratumoral injection) for the treatment of recurrent or metastatic head and neck squamous ...
Biotheus Expanded Their Partnership with Hansoh Pharma for Developing EGFR/cMET Bispecific Antibody-Drug Conjugates
ZHUHAI, China, March 15, 2024 /PRNewswire/ -- On March 14, 2024, Biotheus Inc. (Biotheus), a clinical-stage biotech company focusing on the discovery and development of biologics for oncology and inflammatory diseases, and Hansoh Pharmaceutical Group Co., Ltd. (Hansoh Pharma,03692.HK), China's le...
I-Mab Reports Full Year 2023 Financial Results and Business Update
* Recently announced agreement to divest assets and business operations in China marks an important milestone for the Company; the transaction is expected to close by the end ofMarch 2024 * Uliledlimab (CD73 antibody) on track to file an IND in combination with chemotherapy and checkpoint inh...
D3 Bio Appoints Dr. Antoine Yver as Independent Board Member
SHANGHAI, March 14, 2024 /PRNewswire/ -- D3 Bio, an emerging global biotechnology company that focuses on discovery, development, and registration of innovative cancer drugs, welcomesAntoine Yver, MD. ,MSc. as the new independent board member, effectiveMarch 4, 2024. Dr. Yver has 34 years of e...
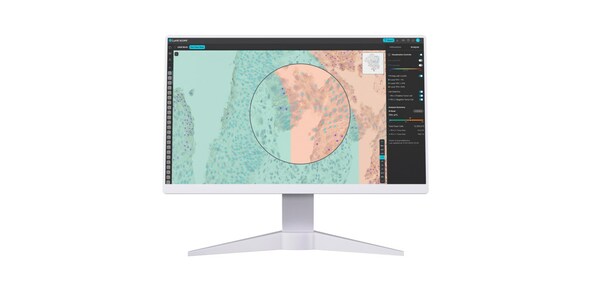
Lunit SCOPE AI Enhances Pathologist Concordance and Accuracy in HER2, ER, and PR Assessment - New Study in Breast Cancer Research
- Lunit SCOPE HER2 and Lunit SCOPE ER/PR significantly improve pathologist concordance and accuracy, paving the way for improved breast cancer molecular subtype analysis for informed decision-making and precision patient care SEOUL, South Korea, March 14, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ...
Study of Kintor's c-Myc Degrader Published in Subsidiary Journal of Nature
SUZHOU, China, March 14, 2024 /PRNewswire/ -- Kintor Pharmaceutical Limited (" Kintor Pharma", HKEX: 9939.HK), a clinical-stage biotechnology company developing innovative small molecule and biological therapeutics, announces that Kintor and its cooperative partner had published an article namedMY...
Sanyou Biopharmaceuticals Launches Advanced Intelligent R&D Service Platform
SHANGHAI, March 12, 2024 /PRNewswire/ -- Sanyou Biopharmaceutical Co., Ltd. is delighted to announce the official launch of the "Sanyou Intelligent Innovative Biologics R&D Service Platform," representing a transformative advancement in biopharmaceutical research and development. Our platform en...
Mabwell to Present Pre-clinical Results at the 2024 American Association for Cancer Research (AACR) Annual Meeting
SHANGHAI, March 12, 2024 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with entire industry chain, announced that it will present results of three preclinical studies as poster presentation at the AACR Annual Meeting to be held inSan Diego, USA, from April 5...
Longbio Pharma Presented Positive Phase 1 Results for LP-003 at 2024AAD
SAN DIEGO, March 12, 2024 /PRNewswire/ -- Longbio Pharma (Suzhou) Co., Ltd. (referred to as "Longbio Pharma"), a leading biotech company dedicated to developing innovative protein treatments for allergy, respiratory, dermatology, hematology, ophthalmology, and other autoimmune and rare diseases, ...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 294 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 291 media titles]
2024-04-22 06:00China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Revenue Surpasses 50 Billion: BlueFocus Accelerates Towards the AI Native Era
[Picked up by 275 media titles]
2024-04-23 15:43INTAMSYS Becomes 3D Printing Equipment Supplier for the WORLDSKILLS LYON 2024 COMPETITION
[Picked up by 272 media titles]
2024-04-24 17:09