Pharmaceuticals
Dr. Lu Xianping, Founder, Chairman of Chipscreen Biosciences named the Chinese mainland winner of the EY Entrepreneur Of The Year™ 2025 awards
SHENZHEN, China, Dec. 29, 2025 /PRNewswire/ -- On December 5, 2025, Dr. Lu
Xianping, Founder, Chairman of Shenzhen Chipscreen Biosciences Co., Ltd, has
been named the Chinese mainland winner of the EY Entrepreneur Of The Year™ 2025
awards.
Adventures in DMPK: Viva Biotech's One-Stop Pharmacology Platform Cross New Modalities
SHANGHAI, Dec. 29, 2025 /PRNewswire/ -- Drug Pharmacokinetics and Pharmacodynamics (PKPD) has become increasingly critical as drug discovery modalities continue to expand across a broader and more diverse therapeutic landscape. Viva Biotech has responded by building an integrated pharmacology pl...
Senhwa Biosciences Aims at Multi-Billion Dollar Global Market with CX-5461 Combined with ADC "Blockbuster" Therapy
TAIPEI and SAN DIEGO, Dec. 28, 2025 /PRNewswire/ -- Following its recent clinical collaboration with the multinational pharmaceutical company BeOne Medicines to explore combination therapy with its marketed PD-1 inhibitor in the challenging field of cold tumor treatment, Senhwa Biosciences, Inc. ...
Harbour BioMed Enters into Long-Term Strategic Collaboration with Lannacheng to Advance Next-Generation Radionuclide Drug Conjugates
CAMBRIDGE, Mass., ROTTERDAM, Netherlands and SHANGHAI, Dec. 28, 2025 /PRNewswire/ -- Harbour BioMed (HKEX: 02142), a global biopharmaceutical company committed to the discovery and development of novel antibody therapeutics in immunology and oncology, today announced that it has entered into a l...
CARsgen Submits Dual IND Applications for Allogeneic BCMA CAR-T Product CT0596
SHANGHAI, Dec. 28, 2025 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on developing innovative CAR T-cell therapies, announced that it has submitted two separate Investigational New Drug (IND) applications to the National Medical Products Administr...
China's First Domestic Anti-CTLA-4 Monoclonal Antibody, Innovent's TABOSUN® (Ipilimumab N01 Injection) Received NMPA Approval
* TABOSUN® (ipilimumab N01 injection) has been approved in combination with TYVYT® (sintilimab injection) for the neoadjuvant treatment of patients with stage IIB-III resectable microsatellite instability-high or mismatch repair deficient (MSI-H/dMMR) colon cancer. * The TABOSUN® and TYVYT® c...
Caliway's Fat Reduction Drug Candidate CBL-514 Completes U.S. FDA IND Submission for Phase 2 Clinical Study in Weight Management, Expanding Development Combined with GLP-1RA-Based Weight-Loss Therapies
* Caliway has completed submission of an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) for a Phase 2 clinical study of CBL-514 for weight management (CBL-0201WR Phase 2). * The study will evaluate CBL-514 in combination with Zepbound® (Eli Lilly) in...
Mabwell Receives IND Clearance for Novel Anti-ST2 Monoclonal Antibody 9MW1911 to Initiate Phase IIa Study
SHANGHAI, Dec. 24, 2025 /PRNewswire/ -- Mabwell (688062.SH), an innovation-driven biopharmaceutical company with a fully integrated industry chain, announced that it has received IND clearance from the U.S. Food and Drug Administration (FDA) for its self-developed anti-ST2 monoclonal antibody (R&...
Nona Biosciences Expands Integrated Discovery-to-Clinical Capabilities Through Strategic Platform Growth
CAMBRIDGE, Mass., Dec. 23, 2025 /PRNewswire/ -- Nona Biosciences, a global biotechnology company advancing biotherapeutic discovery through innovative technology platforms, today announced the expansion of its integrated discovery and development framework to support early clinical development an...
Senhwa Biosciences Highlights AI-Validated Oncology Platform and Strategic Clinical Collaborations Targeting Next-Generation Immuno-Oncology products at Its 2025 Annual Investor Conference
TAIPEI and SAN DIEGO, Dec. 23, 2025 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a clinical stage companies focusing on development of first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today outlined continued progress across its AI-enabled drug developm...
Fapon Biopharma Publishes Pioneering Research in Cell Reports Medicine on FP008, an anti-PD 1 X IL-10M Fusion Protein for Cancer Immunotherapy
DONGGUAN, China, Dec. 23, 2025 /PRNewswire/ -- Fapon Biopharma, a biotech in developing therapeutic biologics including cytokine-antibody fusion proteins and T-cell engagers, announced the publication of pioneering research on FP008, a novel fusion protein inCell Reports Medicine. The peer-review...
Kazia Therapeutics Regains Full Nasdaq Listing Compliance
Restoration of Nasdaq compliance follows $50 million institutional financing and reinforces balance-sheet strength SYDNEY, Dec. 22, 2025 /PRNewswire/ -- Kazia Therapeutics Limited ("Kazia" or the "Company") (NASDAQ: KZIA), a clinical-stage oncology company focused on developing innovative therap...
T-MAXIMUM Pharmaceutical's Allogeneic CAR-T Therapy MT027 Receives FDA IND Clearance to Proceed to Phase II clinical Trial for Recurrent Glioblastoma
BEIJING, Dec. 21, 2025 /PRNewswire/ -- T-MAXIMUM Pharmaceutical announced that its proprietary allogeneic, B7-H3-targeted CAR-T therapy, MT027, has received IND Clearance from the U.S. Food and Drug Administration (FDA) to initiate a Phase II clinical trial for the treatment of recurrent glioblas...
Jacobio Pharma Enters Global Exclusive License Agreement with AstraZeneca for Pan-KRAS Inhibitor JAB-23E73
BEIJING, SHANGHAI and BOSTON, Dec. 21, 2025 /PRNewswire/ -- Jacobio Pharma (1167.HK,) today announced that it has entered an agreement with AstraZeneca for its proprietary Pan-KRAS inhibitor JAB-23E73. AstraZeneca will receive exclusive development and commercialisation rights outside ofChina, wh...
WuXi Biologics Achieves CDP Highest "A" Ratings in Both Climate Change and Water Security
SHANGHAI, Dec. 19, 2025 /PRNewswire/ -- WuXi Biologics (2269.HK) announced that it has been named to the CDP "A" lists for both Climate Change and Water Security for 2025, underscoring its leadership in environmental stewardship and transparent disclosure. Climate Change Leadership Being recogn...
NEW DRUG APPLICATION FOR TINENGOTINIB TABLETS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION
NANJING, China and GAITHERSBURG, Md., Dec. 18, 2025 /PRNewswire/ -- TransThera Sciences Nanjing, Inc. (the "TransThera") announced that the new drug application for Tinengotinib tablets has been accepted by the Center for Drug Evaluation ("CDE")of the National Medical Products Administration ("NM...
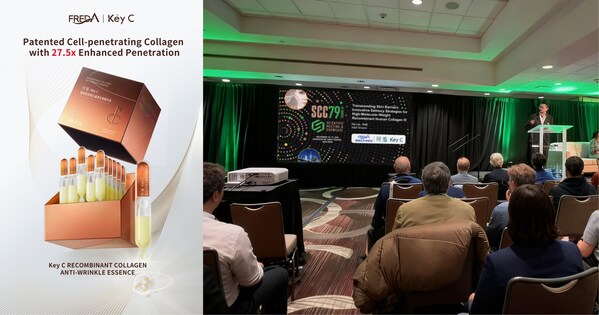
Freda Key C Presents Advanced Recombinant Collagen Delivery Technology at SCC79
Novel Delivery System for High-Molecular-Weight Collagen Advances Anti-Aging Science at the Society of Cosmetic Chemists' Annual Meeting NEW YORK, Dec. 18, 2025 /PRNewswire/ -- Shandong Freda Biotech Co., Ltd. and its medical-aesthetic skincare brand Key C announced the presentation of a novel c...
Nature | Two Phase 3 Clinical Results of Mazdutide (GLP-1/GCG Dual Receptor Agonist) in Chinese Adults with Type 2 Diabetes Have Been Back-to-Back Published in Nature
SAN FRANCISCO and SUZHOU, China, Dec. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncologic, autoimmune, cardiovascular and metaboli...
PRISM BioLab and Talus Bioscience Join Forces to Discover Novel Inhibitors of Transcription Factor and Protein-Protein Interaction Targets
TOKYO, Dec. 17, 2025 /PRNewswire/ -- PRISM BioLab, Co. Ltd. ("PRISM") and Talus Bioscience, Inc. ("Talus Bio") announced today that they entered into a collaboration to discover novel Inhibitors of transcription factor (TF) and protein-protein interaction (PPI) targets. By combining Talus Bio's a...
MEDI&GENE Announces Catalyze Agreement with Lilly to Advance Next-Generation Obesity Therapeutics
SEOUL, South Korea, Dec. 17, 2025 /PRNewswire/ -- MEDI&GENE, a
biopharmaceutical company developing therapeutics for metabolic diseases,
announced today that it has entered into a Catalyze agreement with
Eli Lilly and Company (Lilly) to advance a next-generation therapeutic for
obesity.
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 321 media titles]
2026-02-16 10:00Ascentium Acquires Clara, Expanding into the Abu Dhabi Global Market (ADGM) and Strengthening its Middle East Presence
[Picked up by 310 media titles]
2026-02-12 14:00Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Appier Delivers Record Results Driven by Agentic AI Innovation
[Picked up by 263 media titles]
2026-02-13 17:03Philips Evnia x SEGA: Elevating Immersion for YAKUZA KIWAMI 3 & DARK TIES | Unveiling the 27M2N6501L QD-OLED Monitor - Where Speed Meets Spectacle
[Picked up by 260 media titles]
2026-02-12 15:58