Biotechnology
Insilico Medicine Announces First-in-Human Study for Orally PHD Inhibitor ISM5411 for Inflammatory Bowel Disease (IBD)
* ISM5411 is an oral, PHD-specific inhibitor that facilitates the treatment of IBD by inducing the expression of gut barrier protective genes. * ISM5411 is Insilico Medicine's fifth AI-driven drug program to enter clinical trials, with the first dose of healthy subjects completed inAustralia. ...
CARsgen's CT071 Received IND Clearance from the FDA for Treating Relapsed/Refractory Multiple Myeloma or Relapsed/Refractory Primary Plasma Cell Leukemia
SHANGHAI, Dec. 4, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announces that the U.S. Food and Drug Administration (FDA) has granted Investiga...
Technoderma Medicines Completes Phase 1 Dose Escalation Clinical Trial of TDM-180935 for Atopic Dermatitis
CHENGDU, China, Dec. 4, 2023 /PRNewswire/ -- Technoderma Medicines, Inc. ("the Company"), a clinical stage biopharmaceutical company, is pleased to report that the Company has completed a Phase 1 clinical trial (NCT05525468) of TDM-180935 topical ointment for Atopic Dermatitis (AD). This first cl...
Abbisko Therapeutics Announced the Entry into a Licensing Agreement for Pimicotinib (ABSK021) with Merck
SHANGHAI, Dec. 4, 2023 /PRNewswire/ -- On 4 Dec. 2023, Abbisko Therapeutics announced that it has entered into a licensing agreement with Merck, a leading science and technology company headquartered in Darmstadt,Germany. Under the terms of the agreement, Merck will be granted an exclusive licens...
Mootral introduces new methane reduction technology, doubling efficacy using a well-known natural molecule
Mootral's new platform reduces methane emissions from cows by 50% and can be delivered to both housed and grazing cattle, vastly increasing the opportunity to reduce methane emissions globally LONDON, Dec. 4, 2023 /PRNewswire/ -- Mootral, a British biotech company, announces it has completed the...
Latest Updates of Viva's Portfolio Companies
HONG KONG, Dec. 4, 2023 /PRNewswire/ -- Even with the ever-changing situation, technological innovation is still the most critical component for biopharmaceutical companies' long-term development. This continuous innovation keeps companies up to date and promotes the evolution of R&D and the succ...
Supercell Acquires Exclusive Licensing from Japanese Immunotherapy Leader for Advanced NK Cell Treatment Technology
TAIPEI, Dec. 4, 2023 /PRNewswire/ -- Supercell Biotechnology Corporation commemorates the first anniversary of rebranding from previously-called SinoCell Technologies, due to the company's capital increase by RMT Holding Group. Demonstrating the strategic move at the Healthcare+ Expo Taiwan, the...
Nona Biosciences Announces Collaboration Agreement with Lycia Therapeutics
CAMBRIDGE, Mass., Dec. 3, 2023 /PRNewswire/ -- Nona Biosciences, a global pioneer of technology innovation and antibody discovery and development solutions, announced today it has entered into a collaboration agreement with Lycia Therapeutics, a leader in extracellular protein degradation. Lycia...
Everest Medicines Announces Investigational New Drug Application Acceptance of Zetomipzomib in China
-- Everest plans to contribute to the ongoing global Phase 2b PALIZADE trial of zetomipzomib in active lupus nephritis – -- Zetomipzomib demonstrated clinically meaningful renal responses with a favorable tolerability profile in an earlier Phase 2 trial -- -- Renal and autoimmune diseases are k...
280Bio doses first patient in Phase I clinical trial of TEB-17231, an oral pan-RAS inhibitor in patients with advanced cancers harboring mutations in KRAS, HRAS, or NRAS
SOUTH SAN FRANCISCO, Calif., Dec. 1, 2023 /PRNewswire/ -- 280Bio, Inc., a clinical stage biotechnology company focused on the development of precision oncology medicines, today announced that the first patient was treated with TEB-17231 (YL-17231), a small molecule inhibitor of RAS signaling. 280...
GC Biopharma establishes mRNA production facility in Hwasun, Korea
* Completed the construction of a GMP pilot plant in its Hwasun production plant in Jeollanam-do, Korea * Plans to expand its domain from vaccine development to the field of rare disease treatments by proactively securing mRNA platforms YONGIN, South Korea, Dec. 1, 2023 /PRNewswire/ -- GC Bio...
Innovent Presents Clinical Data Update of IBI351 (KRAS G12C Inhibitor) Monotherapy in Lung Cancer and Colorectal Cancer at ESMO Asia Congress 2023
ROCKVILLE, Md. and SUZHOU, China, Dec. 1, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and...
Cerecin forges strategic partnership with A*STAR's Institute of Molecular and Cell Biology (IMCB) in Singapore to advance R&D in neurological disorders.
Cerecin and IMCB signed a Memorandum of Understanding to collaborate in the areas of neurometabolism, neurodegeneration, metabolic regulators, and brain health and function. SINGAPORE and DENVER, Dec. 1, 2023 /PRNewswire/ -- Cerecin Inc., a clinical-stage biotechnology company at the forefront o...
Everest Medicines Announces Positive Topline Results from Induction Period of Etrasimod Phase 3 Clinical Trial Conducted in Asia for the Treatment of Moderate-to-Severe Active Ulcerative Colitis
--Clinically meaningful and statistically significant results demonstrated for primary endpoint and key secondary endpoints for the 12-week induction period-- --Etrasimod was well-tolerated and its safety profile was consistent with prior studies-- --Everest will advance the late-stage study as...
Biocon Biologics Successfully Completes Integration of Viatris' Biosimilar Business in 31 Countries in Europe
BENGALURU, India, Dec. 1, 2023 /PRNewswire/ -- Biocon Biologics Ltd (BBL), a subsidiary of Biocon Ltd (BSE code: 532523, NSE: BIOCON), today announced that the integration of the Viatris' biosimilars business in 31 European countries has been successfully completed. Following the acquisition of ...
CABIO unveils NeoHMOs™ series at FIE 2023, targeting growing global demand for infant formula
FRANKFURT, Germany, Nov. 30, 2023 /PRNewswire/ -- Fi Europe & Hi 2023 (FIE 2023), the annual European trade show for natural and healthy food and beverage ingredients, is being held inFrankfurt between November 28 and 30, 2023. Over the past 26 years, cumulative visitor count of the prestigiousFi...
Peptone and The Institute of Oncology Research (IOR) Bellinzona Announce Collaborative Research Agreement
Collaboration focused on novel target discovery in prostate cancer LONDON and BELLINZONA, Switzerland, Nov. 30, 2023 /PRNewswire/ -- Peptone, a leader in translational biophysics and the design of novel therapeutics against intrinsically disordered proteins (IDPs), and The Institute of Oncology ...
Phase II OPALESCENCE Study of TLX250-CDx in Triple Negative Breast Cancer to be Presented at SABCS
MELBOURNE, Australia, Nov. 30, 2023 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today advises that the completed Phase II OPALESCENCE investigator-initiated trial (IIT) of its carbonic anhydrase IX (CAIX)-targeting positron emission tomography (PET) imaging candid...
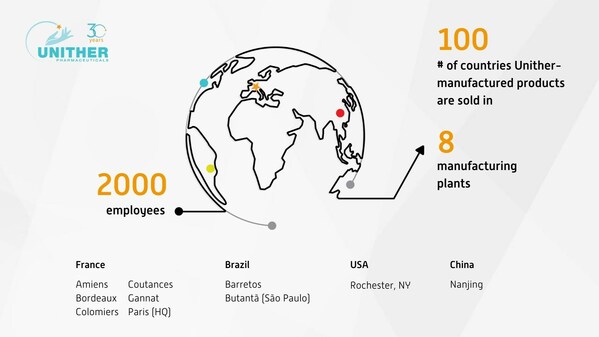
Unither Pharmaceuticals celebrates 30 years of innovation and expertise
PARIS, Nov. 30, 2023 /PRNewswire/ -- Unither Pharmaceuticals, a leading CDMO (Contract Development and Manufacturing Organization), celebrates 30 years in business. In 1993, Unither Pharmaceuticals began operations by acquiring a 17-man pharmaceutical plant that produced 3 pharmaceutical forms. T...
China Pharma Holdings, Inc. Announced the Completion of Third Party Testing of Dry Eye Disease Therapeutic Device
HAIKOU, China, Nov. 29, 2023 /PRNewswire/ -- China Pharma Holdings, Inc. (NYSE American: CPHI) ("China Pharma", or the "Company"), an NYSE American-listed corporation with a fully-integrated specialty pharmaceuticals subsidiary based inChina, today announced that its Dry Eye Disease (DED) therape...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00