Biotechnology
iNtRON, Executes Evaluation License and Option Agreement for SAL200
* iNtRON Grants Basilea exclusive right for preclinical evaluation with an undisclosed payment * Basilea has the option to enter into exclusive License Agreement following the evaluation BOSTON and SEOUL, South Korea, Oct. 30, 2023 /PRNewswire/ -- iNtRON Biotechnology ("iNtRON",www.intodeworl...
CStone Announces NMPA Approval of Sugemalimab for Patients with Relapsed or Refractory Extranodal NK/T-cell Lymphoma, the First Anti-PD-1/PD-L1 mAb Approved for this Indication
* Sugemalimab is the world's first anti-PD-1/PD-L1 monoclonal antibody approved for relapsed or refractory extranodal NK/T-cell lymphoma (R/R ENKTL) indication. * This marks sugemalimab's third indication approved in China following Stage III and IV non-small cell lung cancer (NSCLC) and the ...
AffaMed Therapeutics Announced Positive Top-Line Results from Real-World Study in China Evaluating the Safety and Efficacy of DEXTENZA® in Patients after Cataract Surgery
SHANGHAI, Oct. 31, 2023 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global biotechnology company dedicated to developing and commercializing transformative pharmaceutical, digital and surgical products that address critical unmet medical needs in ophthalmological, neurological and psychia...
Bridge Biotherapeutics Announces Initiation of Phase 1/2 Clinical Trial of BBT-207 in EGFR-Mutant NSCLC
SEONGNAM, South Korea and NEWTON, Mass., Oct. 30, 2023 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330), a South Korean clinical-stage biotech company developing novel drugs for cancer, fibrosis, and inflammation, announced that the company has initiated the Phase 1/2 clinical trial evaluating t...
LifeSpan Vision Ventures Invests in NaNotics
NORWALK, Conn., Oct. 31, 2023 /PRNewswire/ -- LifeSpan Vision Ventures, an investment firm dedicated to longevity biotech, today announced an investment in NaNotics LLC. This preclinical-stage biopharmaceutical company is developing NaNots™, novel subtractive nanoparticles that treat disease by c...
WuXi AppTec Continued Solid Growth in the First Three Quarters of 2023 on Top of an Exceptionally Strong Year in 2022, with Profit Growth Continuously Exceeding Revenue Growth
* Revenue of RMB10,670 Million in the Third Quarter, Single Quarter Revenue Back to overRMB10 Billion; Revenue of RMB29,541 Million in the First Three Quarters, Up 4.0% Year-over-Year; Excluding COVID-19 Commercial Projects, Up 23.4% * Net Profit Attributable to Owners of the Company for the ...
Aquinnah Pharmaceuticals Advances Oral Small Molecule Program Targeting Alzheimer's and Other Tauopathy Diseases
New preclinical data presented at CTAD demonstrates exciting potential for a new drug approach for Alzheimer's disease, reducing brain tau pathology by ~70% CAMBRIDGE, Mass., Oct. 30, 2023 /PRNewswire/ -- Aquinnah Pharmaceuticals announced preclinical research findings for a novel therapeutic des...
First Patient Enrolled in Phase III Clinical Trial of Boan Biotech's Nivolumab Injection
YANTAI, China, Oct. 30, 2023 /PRNewswire/ -- Boan Biotech (6955.HK) announced today that the first patient in Phase III clinical trial of its Nivolumab Injection (BA1104) inChina has been enrolled. BA1104 is the first biosimilar to Opdivo® to undergo a Phase III study in China. Nivolumab is a mo...
Henlius Announces NMPA Approvals of Two ADC Candidates for IND
SHANGHAI, Oct. 27, 2023 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696. HK) announced that the investigational new drug (IND) applications of HLX42 for Injection, a novel EGFR-targeting antibody-drug conjugate (ADC) as well as HLX43, a novel PD-L1-targeting ADC, have been approved by the Na...
Henlius Deepens Collaboration with Intas to bring Henlius' Novel anti-PD-1 mAb Serplulimab to Europe and India
* The footprint of serplulimab now includes the United States, Europe, Southeast Asia, MENA, and India * Intas to develop and commercialise serplulimab in Europe and India; Henlius to receive €42 million upfront payment, double-digit royalties and up to €143 million in regulatory and commerc...
Henlius Deepens Collaboration with Intas to bring Henlius' Novel anti-PD-1 mAb Serplulimab to Europe and India
* The footprint of serplulimab now includes the United States, Europe, Southeast Asia, MENA, and India * Intas to develop and commercialise serplulimab in Europe and India; Henlius to receive €42 million upfront payment, double-digit royalties and up to €143 million in regulatory and commerci...
Chime Biologics and Hope Medicine Enter Manufacturing Agreement to Speed up the Launch of First-in-class Antibody Drug HMI-115 Targeting Endometriosis and Androgenic Alopecia
* Chime Biologics to support the late-stage clinical study and to provide global commercial manufacturing services forHope Medicine's first-in-class monoclonal antibody drug, HMI-115. * The first-in-class mAb will benefit endometriosis and androgenetic alopecia patients. SHANGHAI, Oct. 26, 20...
Clarity and PSI kick off SAR-bisPSMA Phase III
SYDNEY, Oct. 26, 2023 /PRNewswire/ -- Clarity Pharmaceuticals (ASX: CU6) ("Clarity"), a clinical stage radiopharmaceutical company with a mission to develop next-generation products that improve treatment outcomes for children and adults with cancer, and PSI CRO AG ("PSI"), a global contract rese...
YS Biopharma Announces Receipt of Nasdaq Notice
GAITHERSBURG, Md., Oct. 26, 2023 /PRNewswire/ -- YS Biopharma Co., Ltd. (NASDAQ: YS) ("YS Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for infectious disea...
HanAll Biopharma Reports Third Quarter 2023 Financial Results and Provides a Business Update
* Sales recorded KRW 33 billion in Q3 2023, an 11 percent increase from the same period in 2022, due to the sustained growth of major products. * 'HL161ANS', HanAll's second anti-FcRn antibody, demonstrated best-in-class potential through the initial outcome of Phase 1 study. * 'HL192', a Nu...
Fapon, LASCCO, Abionic Formed Strategic Collaboration for PSP Sepsis Diagnosis in China
LAUSANNE, Switzerland, Oct. 26, 2023 /PRNewswire/ -- Fapon, a leading life sciences company, entered into a strategic cooperation agreement with two Swiss biotech companies LASCCO SA and Abionic SA, that granted Fapon an exclusive license to utilize the pancreatic stone protein (PSP) biomarker fo...
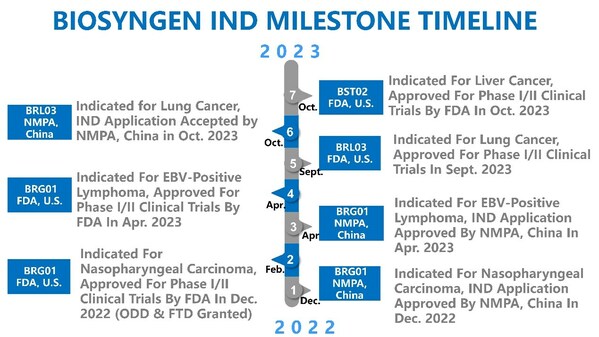
Biosyngen's BST02, the World's First TIL Therapy for Liver Cancer, is Granted an IND Approval by FDA
SINGAPORE, Oct. 26, 2023 /PRNewswire/ -- On October 26, 2023, Biosyngen's TIL therapy BST02 for liver cancer was granted an approval for clinical trial by the US FDA. BST02, a breakthrough product in the field of cell and gene therapy, represents the world's first TIL therapy designed for the tre...
Bridge Biotherapeutics Announces Positive Recommendation from the Independent Data Monitoring Committee of the BBT-877 Phase 2a Study in Idiopathic Pulmonary Fibrosis
* The independent data monitoring committee (IDMC) recommended the continuation of the BBT-877 Phase 2a trial based on the initial clinical data from the first 20 participants dosed with theinvestigational product * No safety concerns were raised based on the evaluation of the data presented ...
First Patient Dosed for MRCT Phase 3 Study on First-Line LS-SCLC of Henlius Anti-PD-1 mAb Serplulimab in Europe
SHANGHAI, Oct. 25, 2023 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the first patient inEurope has been dosed in the international multi-centre phase 3 clinical trial (NCT05353257) of the company's self-developed anti-PD-1 mAb HANSIZHUANG (serplulimab) in combination ...
FDA Meeting Feedback Puts Turn Biotechnologies on Track to be First Longevity Company Taking Cell Rejuvenation Therapy to Clinic
* FDA INTERACT meeting reviews Turn Bio's ERA™ and eTurna™ technologies, approach and plans, paving way toward its IND * Company continues to pace industry's cell regeneration therapy progress MOUNTAIN VIEW, Calif., Oct. 25, 2023 /PRNewswire/ -- Turn Biotechnologies, a cell rejuvenation compa...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00