Biotechnology
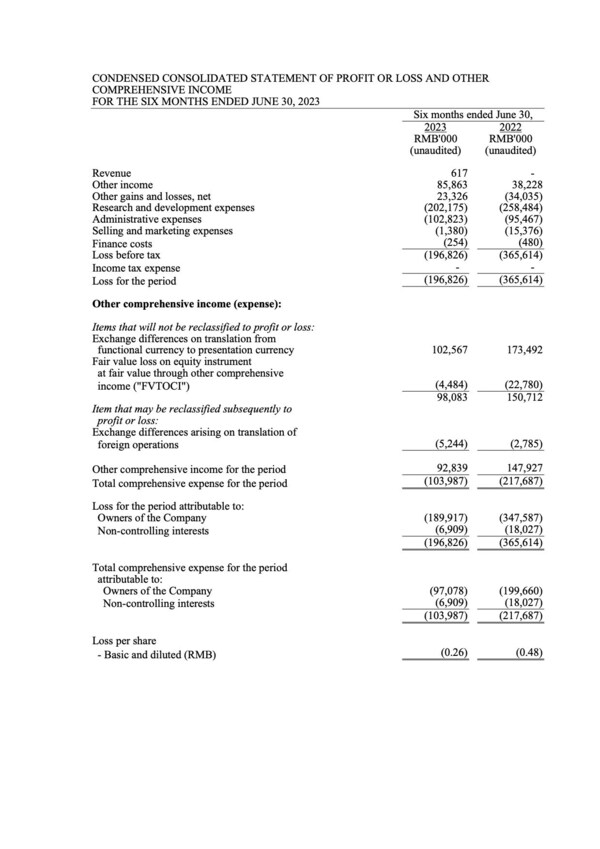
Brii Biosciences Provides Corporate Updates and Reports 2023 Interim Results
First patient dosed in a PEG-IFN-α controlled BRII-835 + PEG-IFN-α combination Phase 2 study for HBV functional cure First of several studies to investigate the potential of BRII-179 in enriching patients with strong intrinsic anti-HBsAg responses for curative treatments to start before the end ...
Paradigm Therapeutics Acquires Late Stage "Breakthrough Therapy" Designated Therapy for Treatment of All Subtypes of Epidermolysis Bullosa (EB)
'Topical Therapy for Treating the Wounds and Lesions on the Entire Skin Surface' MT PLEASANT, S.C., Aug. 22, 2023 /PRNewswire/ -- Paradigm Therapeutics Inc., a biopharmaceutical company, today announced the completion of the acquisition of the worldwide rights of SD-101, a topical whole-body trea...
Asieris Pharmaceuticals Unveils 2023 Semi-Annual Report, Demonstrating Initial Success of Specialty Pharma Strategy and Multi-Product Synergistic Commercialization
SHANGHAI, Aug. 22, 2023 /PRNewswire/ -- Asieris Pharmaceuticals (Stock Code: 688176.SH) – a global innovative pharmaceutical company specializing in the discovery, development and commercialization of innovative drugs that treat genitourinary (GU) tumors and other related diseases – today release...
Sanyou Biopharmaceuticals and Sinorda Biomedicine signed Strategic Cooperation Agreement for Advancing Innovative Drug Research and Development
SHANGHAI, Aug. 22, 2023 /PRNewswire/ -- Sanyou Biopharmaceuticals (Shanghai) Co., Ltd. (referred to as "Sanyou" hereafter) and Sinorda Biomedicine (referred to as " Sinorda " hereafter) have officially signed a strategic cooperation agreement to advance the innovative bispecific antibody drug pro...
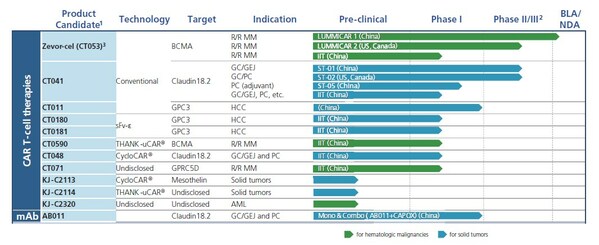
CARsgen Announced 2023 Interim Results
SHANGHAI, Aug. 22, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced its 2023 Interim Results. Business Highlights * Collaboration agr...
Kintor Pharma Announces Completion of Patient Enrollment in Phase II Clinical Trial of GT20029 for Treatment of Androgenetic Alopecia in China
SUZHOU, China, Aug. 22, 2023 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma", HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, announced that the company has completed the enrollment of 180 patients for the Phase...
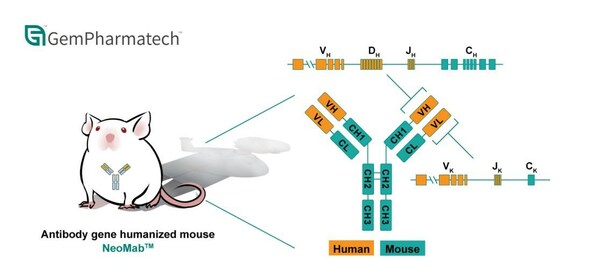
Introducing NeoMab™: GemPharmatech's Innovative Antibody Gene Humanized Mouse Model
SAN DIEGO, Aug. 21, 2023 /PRNewswire/ -- GemPharmatech is proud to announce the launch of NeoMab™, our independently developed fully antibody gene humanized mouse model, after four years of rigorous research and validation. NeoMab™ is specifically designed to meet the burgeoning therapeutic antib...
Lunit Backs the Potential of Tertiary Lymphoid Structures Analysis as an Emerging Biomarker for Lung Cancer Treatment - to be presented at WCLC 2023
- A collaborative study demonstrates that AI analysis of Tertiary Lymphoid Structures (TLS) in tumors can predict treatment response in non-small cell lung cancer patients SEOUL, South Korea, Aug. 21, 2023 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for canc...
CanariaBio Achieves Significant Milestone with FDA's Orphan Drug Designation for MAb-AR20.5 Targeting Pancreatic Cancer
The investigational monoclonal antibody becomes the first to target Mucin 1 (MUC1) and receive this designation. PYEONGTAEK, South Korea, Aug. 21, 2023 /PRNewswire/ -- CanariaBio Inc., a clinical-stage biopharmaceutical company focused on the development and commercialization of innovative immun...
Hummingbird Bioscience to Present Poster on HMBD-001 Combination Strategy for Squamous Non-Small Cell Lung Cancer at World Conference on Lung Cancer 2023
HOUSTON and SINGAPORE, Aug. 21, 2023 /PRNewswire/ -- Hummingbird Bioscience, a data-driven precision biotherapeutics company discovering and developing transformative biologic medicines for hard-to-treat diseases, today announced an upcoming poster presentation at the IASLC 2023 World Conference ...
3Z and biotx.ai Forge Partnership to Advance ADHD Drug Development Using AI Modelling
REYKJAVIK, Iceland and BERLIN, Aug. 18, 2023 /PRNewswire/ -- Reykjavik-based drug discovery company, 3Z, andGermany/US-based causal AI firm, biotx.ai, have joined forces in a strategic partnership aimed at revolutionizing ADHD drug development. By leveraging advanced AI modelling techniques, the ...
Inmagene's anti-OX40 mAb demonstrated an extended half-life and a favorable safety profile in a Phase I study
* IMG-007 has the potential to provide Q12W or less frequent dosing based on an extended half-life * IMG-007 has demonstrated a favorable safety profile, consistent with a silenced ADCC function via bioengineering * IMG-007 is being evaluated in two global proof-of-concept trials SAN DIEGO,...
QiLu Pharmaceutical's Iruplinalkib Phase III INSPIRE Study Researched Primary Endpoint and was Selected for 2023 WCLC Oral Presentation
JINAN, China, Aug. 18, 2023 /PRNewswire/ -- August 16, 2023, at the 2023 World Conference on Lung Cancer (WCLC), the complete list of selected abstracts was announced, among which QiLu Pharmaceutical's iruplinalkib phase III clinical trial (INSPIRE) results were chosen for oral presentation at th...
Thrombotic AEs of Hemlibra (emicizumab) were 2.8 times more frequent than those of FVIII replacements.
* Analyzed the FDA FAERS data…to compare the thrombotic AEs of Hemlibra vs. FVIII replacements. YONGIN, South Korea, Aug. 17, 2023 /PRNewswire/ -- GC Biopharma (006280.KS), a South Korean biopharmaceutical company, announced on August 17, 2023 that it has presented the results of analyzing and...
Neurophth Announces First Patient Dosed in Phase I/II Clinical Trial of Second Gene Therapy
WUHAN, China and SAN DIEGO, Aug. 17, 2023 /PRNewswire/ -- Neurophth Therapeutics, Inc. ("Neurophth") announced today that the first patient has been dosed in the international multi-region, multi-center Phase I/II clinical trial for the treatment of Leber hereditary optic neuropathy caused byND1 ...
Minghui Pharmaceutical Inc. Announces First Patient Enrollment in the Phase 3 Clinical Study of MH004 Cream for Treatment of Mild to Moderate Atopic Dermatitis
SHANGHAI, HANGZHOU, China and WILMINGTON, Del., Aug. 17, 2023 /PRNewswire/ -- Minghui Pharmaceutical, Inc., a leading clinical-stage biopharmaceutical company, today announced the successful enrollment of the first patient in the phase 3 clinical study for MH004 Cream targeting mild to moderate a...
I-Mab Provides Mid-Year 2023 Financial Results, Business and Corporate Updates
* Significant progress made year-to-date on key clinical assets: ‐ Uliledlimab (CD73 antibody): Encouraging early results were presented at ASCO 2023 ‐ Givastomig (Claudin 18.2 x 4-1BB bispecific antibody): Topline Phase 1 data with promising early efficacy signals, including pat...
Novo Holdings participates in $290m strategic financing of Sangon Biotech
COPENHAGEN, Denmark, Aug. 17, 2023 /PRNewswire/ -- Novo Holdings, a leading international life science investor, today announced it has participated in a $290 million (USD) strategic financing round of Sangon Biotech. Sangon is a leading provider of life science tools and services, enabling scient...
Innovent Announces the NMPA Approval of SINTBILO® (Tafolecimab Injection) for the Treatment of Adult Patients with Primary Hypercholesterolemia and Mixed Dyslipidemia
ROCKVILLE, Md. and SUZHOU, China, Aug. 17, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology an...
Oncoclínicas Group generated record earnings in the second quarter of 2023, R$309 million and a net profit of R$35 million
Efficient management of working capital explains a large part of the cash flow performance. Net income grew 51% (31% organically) while the Ebitda Ex-LTIP (excluding only the non-cash impact of the long-term incentive plan) reached R$268 million, 109% greater than the same period last year SÃO PA...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00