Biotechnology
BioDuro-Sundia Announces Formation of Scientific Advisory Board
IRVINE, Calif., March 28, 2023 /PRNewswire/ -- BioDuro-Sundia, a leading drug discovery, development and commercial services organization backed by Advent International, announced today the establishment of a Discovery Research Scientific Advisory Board (SAB) with the appointment of Andrew Thomas...
BIORCHESTRA Pens a Pact Worth up to $861M for Central Nervous System(CNS)-Targeted Polymeric Nanoparticle for Intravenous Delivery of Nucleic Acid Therapies
Exclusive Research and Option Agreement With Global Biopharmaceutical Company to Leverage BIORCHESTRA Targeting Technology Platform -BDDS™ - to Develop Nucleic Acid Therapies for Treatment of Neurological Disorders in Multiple Targets CAMBRIDGE, Mass. and DAEJEON, South Korea, March 27, 2023 /PR...
YS Biopharma's PIKA Recombinant COVID-19 Vaccine Demonstrates Superior Antibody Neutralization Responses Compared to Inactivated COVID-19 Vaccine in Phase II Head-to-Head Clinical Study
* The trial met its primary endpoint of superior immunogenicity of PIKA COVID-19 Vaccine vs inactivated COVID-19 vaccine, measured by GMT of neutralizing antibody against Omicron virus on Day 14, with statistical significance (95%CI: 2.1, 3.4, P<0.0001) based on interim data analysis * The tr...
Noul Co. Ltd - The RIGHT Foundation Commences Research and Development for Supporting Malaria Control
* Aim to accelerate global business and access to the public market based on reliable research results YONGIN, South Korea, March 27, 2023 /PRNewswire/ -- Noul Co. Ltd (CEO :David Lim) announced that it has signed an agreement with The RIGHT Foundation to conduct a global clinical studies to de...
Pharming announces US FDA approval of Joenja® (leniolisib) as the first and only treatment indicated for APDS
APDS (activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome) is a rare and progressive primary immunodeficiency Joenja® is a targeted treatment of APDS for adult and pediatric patients 12 years of age and older Joenja® is expected to launch in the US in early April Pharming will host a con...
ProfoundBio Highlights Broad Antitumor Activity in Preclinical Models and Trial-in-Progress for PRO1184 at AACR 2023 Annual Meeting
WOODINVILLE, Wash. and SUZHOU, China, March 24, 2023 /PRNewswire/ -- ProfoundBio, a clinical stage biotechnology company focused on the development of novel antibody-based therapeutics with curative potential, will present two posters from its lead program, PRO1184, a folate receptor alpha (FRα)-...
World TB Day 2023: Illumina and GenoScreen Launch Next Generation Sequencing Innovation to Eliminate Tuberculosis in Africa
Transformative Next Generation Sequencing (NGS) Innovation expands access to genomic testing and enables personalized medicine for patients with Tuberculosis (TB) CAPE TOWN, South Africa, March 24, 2023 /PRNewswire/ -- Illumina Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and array-bas...
Brii Biosciences Provides Corporate Update and Reports Full-Year 2022 Financial Results
Company maintains priority focus on clinical programs to develop a novel f unctional cure for hepatitis B viral (HBV) infection and a potential first-of-its-kind treatment for postpartum depression (PPD) and major depressive disorders (MDD) Multiple Phase 2a proof-of-concept (POC) clinical data r...
EMA Validates Marketing Authorization Application for Henlius' HANSIZHUANG (Serplulimab)
* HANSIZHUANG (serplulimab) is the first anti-PD-1 mAb for the first-line treatment of small cell lung cancer (SCLC) * The EC and the FDA previously granted Orphan Drug Designations (ODDs) for HANSIZHUANG in SCLC * HANSIZHUANG is approved in China for microsatellite instability-high (MSI-H)...
Northumbria Healthcare NHSFT and C-POLAR Technologies combine innovation in widely acclaimed hub
This partnership will create infection-resilient environments as C-POLAR
captures, inactivates, and eradicates up to 99.99% of viruses, bacteria, and
fungi
HONG KONG, March 23, 2023 /PRNewswire/ -- C-POLAR Technologies, Inc.
AnPac Bio-Medical Science Granted Continued Listing by NASDAQ Hearing Panel, Subject to Meeting the Equity Rule on or before July 12, 2023
NEW YORK, March 23, 2023 /PRNewswire/ -- AnPac Bio-Medical Science Co., Ltd. (the "Company") (NASDAQ: ANPC), a company with operations inthe United States andChina focused on early cancer screening and detection and plans to enter into the operation of a business-to-business e-commerce food platf...
Everest Medicines Announces Signing of an MOU for Strategic Cooperation with Guangdong Academy of Medical Sciences in Renal Diseases
GUANGZHOU, China, March 23, 2023 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the development, manufacturing and commercialization of innovative medicines and vaccines, announced today that it has signed an MOU for comprehen...
Ascentage Pharma Announces 2022 Annual Results Including Strong Sales of Olverembatinib and Steady Progress in Transition Towards Biopharma
SUZHOU, China and ROCKVILLE, Md., March 22, 2023 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced its annual results for the full year 2022. During...
Daewoong Pharmaceutical's Envlo to enter the global market in full swing with filing for product license in three ASEAN countries
* Submitted an NDA to Indonesia, Philippines and Thailand * Signed the export contract in Brazil and Mexico in last February * Aiming to enter 50 countries by 2030 SEOUL, South Korea, March 22, 2023 /PRNewswire/ -- Daewoong Pharmaceutical began the advance into the global market in full swin...
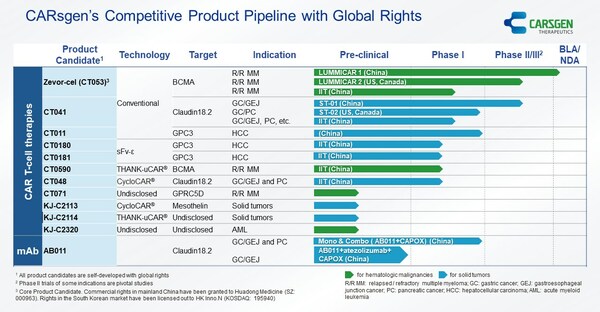
CARsgen Announced 2022 Annual Results and Business Updates
SHANGHAI, March 22, 2023 /PRNewswire/ -- CARsgen Therapeutics Holdings Limited (Stock Code: 2171.HK), a company focused on innovative CAR T-cell therapies for the treatment of hematologic malignancies and solid tumors, announced its 2022 Annual Results. Business Highlights * Zevor-cel (CT053)...
Biosion bispecific antibody therapies to be featured at the American Association for Cancer Research (AACR) annual meeting 2023
NEWARK, Del., March 22, 2023 /PRNewswire/ -- Biosion USA, Inc. (Biosion), a global R&D biotechnology company, today announced the upcoming presentations of non-clinical data from its oncology pipeline, including BSI-507: anti-PD1ⅹanti-PVRIG bispecific antibody and BSI-508: anti-PD1ⅹanti-CD47 bis...
Jacobio announces clinical collaboration to evaluate CD73 monoclonal antibody JAB-BX102 in combination with KEYTRUDA® (pembrolizumab) for patients with cancer
BEIJING, SHANGHAI and BOSTON, March 22, 2023 /PRNewswire/ -- Jacobio Pharma (1167.HK) announced it has entered into a clinical collaboration with Merck & Co., Inc.,Rahway, NJ, USA to evaluate the combination of Jacobio's CD73 monoclonal antibody JAB-BX102 in combination with Merck & Co., Inc., Ra...
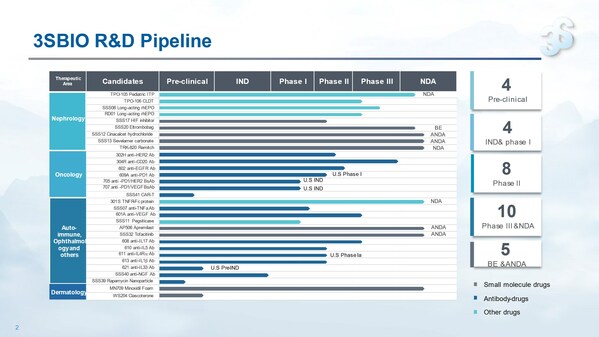
3SBio announces 2022 annual results, with revenue rising 7.5% year on year and normalized net profit attributable to parents jumping 25.2%
HONG KONG, March 22, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today announced its 2022 annual results. In 2022, 3SBio's core biopharmaceutical products posted robust growth, hair health business achieved blistering performance, and contract development and ...
DEBIOPHARM ANNOUNCES LAUNCH OF THE PHASE 1/2 GaLuCi™ STUDY FOR ITS CA IX-TARGETED RADIOPHARMACEUTICAL PROGRAM
* Debiopharm is developing personalized radiotherapy through a theranostic approach, combiningdiagnostic imaging (Debio 0328 a gallium-labelled imaging tool) and therapeutic components (Debio 0228, a lutetium-labelled radioligand), thus allowing the pre-identification and treatment of patients ...
280Bio Provides Update on the KRAS Inhibitor TEB-17231 at the 2023 AACR Annual Meeting
A broadly acting KRAS Inhibitor, TEB-17231, robustly blocks tumor growth and overcomes KRASG12C inhibitor mediated resistance SHANGHAI, March 21, 2023 /PRNewswire/ -- 280Bio, Inc. a clinical stage biotechnology company, focused on the development of precision oncology medicines, today announced ...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00