Biotechnology
PharmAbcine Announces Collaboration Agreement with MSD to Evaluate Anti-VISTA Antibody PMC-309 in Combination with KEYTRUDA® (pembrolizumab)
DAEJEON, South Korea, Dec. 12, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next-generation antibody therapeutics, announced today that the Company has entered into a clinical collaboration agreement with MSD (Merck & Co...
Asieris'Bladder Cancer Diagnostic Drug Hexvix® Completed Dosing of First Patient in Real-World Clinical Study
SHANGHAI, Dec. 12, 2022 /PRNewswire/ -- Asieris Pharmaceuticals (688176), a global biopharma company specializing in discovering, developing and commercializing innovative drugs for the treatment of genitourinary tumors and other related diseases, announced that Hexvix®, a drug used for bladder c...
JW Therapeutics Presents Latest Clinical Data on Carteyva® in Follicular Lymphoma and Mantle Cell Lymphoma at the 64th ASH Annual Meeting
SHANGHAI, Dec. 12, 2022 /PRNewswire/ -- JW Therapeutics (HKEX: 2126), an independent and innovative biotechnology company focusing on developing, manufacturing and commercializing cell immunotherapy products, presented the latest clinical data on Carteyva® (relmacabtagene autoleucel injection) in...
HitGen Announces Research Agreement with Nitrase Therapeutics Focused on DNA-Encoded Library Based Drug Discovery
CHENGDU, China, Dec. 11, 2022 /PRNewswire/ -- Shanghai Stock Exchange listed company HitGen Inc. ("HitGen", SSE: 688222.SH) today announced that it has entered into a research agreement with Nitrase Therapeutics, Inc. ("Nitrase"), a biopharmaceutical company deploying its NITROME platform to buil...
Gracell Biotechnologies Presents Clinical Data for FasTCAR-T GC012F for High Risk, Newly Diagnosed Multiple Myeloma Demonstrating 100% Overall Response Rate
BCMA/CD19 dual-targeting FasTCAR-T GC012F data from ongoing clinical trial presented at 64th American Society of Hematology Annual Meeting and Exposition SAN DIEGO and SUZHOU and SHANGHAI, China, Dec. 10, 2022 /PRNewswire/ -- Gracell Biotechnologies Inc. ("Gracell" or the "Company", NASDAQ: GRCL)...
Goodwin Biotechnology Inc. is now GBI, as Part of their Rebranding Initiative on their 30-Year Anniversary!
PLANTATION, Fla., Dec. 10, 2022 /PRNewswire/ -- Goodwin Biotechnology, Inc. announces its new name, GBI, as part of a rebranding initiative to commemorate its 30-year anniversary. This includes a new name, logo and website to reflect its evolution from an early-stage clinical manufacturing Contra...
Treadwell Therapeutics Announces A Presentation at the 2022 SABCS Annual Meeting Featuring a Clinical Trial Update on CFI-402257, a Best-in-Class TTK inhibitor
NEW YORK, Dec. 9, 2022 /PRNewswire/ -- Treadwell Therapeutics, a clinical-stage biotechnology company developing novel medicines for highly aggressive cancers, today announced that data presented on the ongoing CFI-402257-CL-001 clinical study of the Company's CFI-402257 program in advanced solid...
Shenzhen Holds 2022 Global Investment Promotion Conference to Build a Magnet for International Investors
SHENZHEN, China, Dec. 8, 2022 /PRNewswire/ -- Shenzhen welcomes outstanding investors from all over the world. The 2022 Shenzhen Global Investment Promotion Conference will be held inShenzhen, China on December 9. A number of major projects in the fields of information technology, artificial int...
Antengene Announces HREC Approval in Australia for the Phase I Trial of ATG-022 (Claudin 18.2 ADC) in Patients with Advanced or Metastatic Solid Tumors
* Discovered in-house by Antengene's R&D team, ATG-022 is an antibody-drug-conjugate targeting the Tumor Associated Antigen (TAA) Claudin 18.2 * The Phase I trial is designed to evaluate the safety, pharmacology, and preliminary efficacy of ATG-022 as a monotherapy in patients withadvanced or...
SK Biopharmaceuticals' Partner Angelini Pharma Launches Cenobamate in France
Cenobamate (ONTOZRY®), for the treatment of uncontrolled focal-onset seizures in adults, is now available in 5 major European countries –Germany, the UK, Italy, Spain, France – and 10 other key markets PANGYO, South Korea, Dec. 8, 2022 /PRNewswire/ -- SK Biopharmaceuticals, an innovative global ...
ReviR Therapeutics to Present at RNA Targeted Drug Discovery Summit
SAN FRANCISCO, Calif., Dec. 8, 2022 /PRNewswire/ -- ReviR Therapeutics (ReviR), a biotechnology company developing small molecule therapies targeting RNA, today announced an upcoming presentation at the 5th Annual RNA Targeted Drug Discovery conference taking placeDecember 13-15, 2022 in Boston, ...
LISCure's Phase 2 Drug Candidate Granted Orphan Drug Designation (ODD) from US FDA
First Orphan Drug Designation (ODD) in the field of microbiome-based treatment for metabolic diseases SEOUL, South Korea, Dec. 8, 2022 /PRNewswire/ -- LB-P8, a novel drug being developed by LISCure Biosciences, Inc. (LISCure) as a treatment for Primary Sclerosing Cholangitis (PSC), has been desi...
PharmAbcine to Participate in the 41th Annual J.P. Morgan Healthcare Conference and Biotech Showcase™ 2023 during JPM Week
DAEJEON, South Korea, Dec. 8, 2022 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks), a clinical-stage biotech company focusing on the development of next-generation antibody therapeutics, announced today that the Company is invited to participate in upcoming 41th Annual J.P. Morgan Healthcare ...
CABIO Showcases the Latest Research Results and Innovative Solutions at Food Ingredients Europe 2022
PARIS, Dec. 8, 2022 /PRNewswire/ -- On December 6, 2022, CABIO Biotech (Wuhan) Co., Ltd (CABIO), a leading Chinese biotechnology company, took part in the three-day Food Ingredients Europe 2022 (Fi Europe) held inParis, France. Fi Europe has been recognized as the industry's highest level interna...
Createrna Announces Positive Top-Line Results from Phase 1 Clinical Trials of QR052107B Tablet in Healthy Volunteers
* QR052107B Tablet was safe and well tolerated in healthy volunteers across all tested doses, with no taste related adverse event at the anticipated therapeutic doses * Createrna initiated a clinical Phase 2 trial to study QR052107B Tablet at once-a-day (QD) dose in the treatment of refract...
PGTG successfully develops SGF bone-derived factor to restore the skeletal system
BEIJING, Dec. 7, 2022 /PRNewswire/ -- The bone-derived skeletal growth factor (SGF), jointly developed by a research facility under the direction of leading biochemist, academician and researcher Zhao Yufen and theAnhui-based research center of one of the world's health industry leaders People's ...
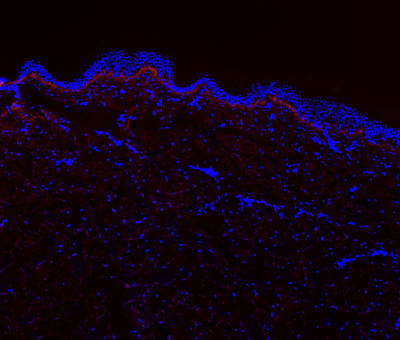
Turn Biotechnologies Changes Paradigm in Skin Rejuvenation
* Data unveiled at four industry conferences suggest a single ERA treatment may be more effective than combination therapies used today * Biomarker analysis demonstrates ERA's regenerative impact on fibroblast proliferation, collagen VII production * eTurna™ lipid-based delivery platform p...
Newtown's Apple to Highly Saturated Global Molecular Market, TIANGEN TGuide S16 Automated Nucleic Acid Extractor Showcased at Cell Bio 2022
WASHINGTON, Dec. 6, 2022 /PRNewswire/ -- TIANGEN, a high-tech biological enterprise, demonstrated its revolutionary nucleic acid extraction solution at Cell Bio 2022, the joint meeting of the American Society for Cell Biology (ASCB) and European Molecular Biology Organization (EMBO), fromDec 4 to...
Akeso Inc. Announces Collaboration and License Agreement for Up to US$5 Billion with Summit Therapeutics to Accelerate Global Development and Commercialization of its Breakthrough Bispecific Antibody, Ivonescimab (PD-1/VEGF)
* Akeso Inc. to out-license to Summit Therapeutics exclusive rights to ivonescimab (PD-1/VEGF) for the development and commercialization inthe United States, Canada, Europe, and Japan. Akeso Inc. will retain development and commercialization rights for the rest of the world includingChina. * ...
Daewoong Pharmaceutical announces success in developing a new antidiabetic medication and its aims to enter the market in over 50 countries by 2030
* Delivers new drug for two consecutive years by obtaining product license on "Envlo", Korea's 36th new drug from the Ministry of Food and Drug Safety * Plans to enter the type 2 diabetes global market worth $71.4 billion * Provides new and broad treatment options for patients with insufficie...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 299 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 295 media titles]
2024-11-26 13:00TAILG's First Flagship Store in Indonesia Grandly Opens
[Picked up by 283 media titles]
2024-11-22 21:59MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 278 media titles]
2024-11-25 10:00