Biotechnology
I-Mab Announces Partnership Agreement to Localize Manufacturing and Accelerate Commercialization of Innovative Biologics Drugs
SHANGHAI and GAITHERSBURG, Md., Jan. 28, 2022 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical-stage biopharmaceutical company committed to the discovery, development, and commercialization of novel biologics, announced the signing of a partnership agreement with the Hangzhou Qian...
Alterity Therapeutics Announces Publication Demonstrating that ATH434 is Neuroprotective in Animal Model of Parkinsonian Disorder in the Journal of Parkinson's Disease
MELBOURNE, Australia, Jan. 28, 2022 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative diseases, today announced that data in an animal model of Multiple S...
Ready to Enter the Middle East Market, Vazyme Showcases Its COVID-19 Testing Solutions at Medlab Middle East 2022
DUBAI, UAE, Jan. 28, 2022 /PRNewswire/ -- Leading China-based biotech company Vazyme (688105.SH) participated in the 2022 edition of Medlab Middle East in the Dubai World Trade Centre (DWTC) fromJanuary 24th to 27th, a leading laboratory and flagship exhibition and conference that brings together...
Samsung Biologics reaches agreement with Biogen to acquire full ownership of Samsung Bioepis
* Agreement with Biogen to buyout Biogen's (50% -1 share) stake in Samsung Bioepis Joint Venture for up to USD$2.3 billion * Transaction projected to be accretive to earnings in 2022 and thereafter, fully capitalizing on the high growth potential of Samsung Bioepis * Expected to accelerate g...
HaemaLogiX and Lonza Collaborate to Manufacture KappaMab, a Multiple Myeloma Drug Candidate
* Lonza to manufacture drug substance for clinical supply of HaemaLogiX's lead multiple myeloma drug candidate, KappaMab, at its new state-of-the-art facility inGuangzhou (CN) * HaemaLogiX will leverage Lonza's regulatory expertise, global manufacturing footprint, and extensive experience in ...
FDA Grants Breakthrough Therapy Designation for Dizal Pharmaceutical's DZD9008 in Patients with Locally Advanced or Metastatic Non-Small Cell Lung Cancer Harboring EGFR Exon20 Insertion
SHANGHAI, Jan. 27, 2022 /PRNewswire/ -- Dizal Pharmaceutical Co., Ltd. (SHEX:688192) ("Dizal"), today announced that the U.S. Food and Drug Administration ("FDA") has granted Breakthrough Therapy Designation toDZD9008 (Sunvozertinib) for the treatment of patients with locally advanced or metasta...
Brii Bio Appoints Karen D. Neuendorff as Chief People Officer and Head of Human Resources
DURHAM, N.C. and BEIJING, Jan. 27, 2022 /PRNewswire/ -- Brii Biosciences
Exyte Closes 2021 with Record Order Intake
- Order intake FY 2021 of over €8 billion, more than doubling YOY - Semiconductor and battery boom are main drivers for increased order intake - Outlook continues to be very positive STUTTGART, Germany, Jan. 27, 2022 /PRNewswire/ -- Exyte, a global leader in the design, engineering, and delivery ...
Appendix 4C - Q2 FY22 Quarterly Cash Flow Report
Highlights: * NZ's regulator Medsafe authorizes Phase 2 clinical trial for ATH434; more jurisdictions to follow * New poster presentation and publications provide further evidence of potential of ATH434 to treat neurodegenerative diseases * New US composition of matter patent secures exclus...
I Peace's Cell Manufacturing Facility "Peace Engine Kyoto" now registered as U.S. FDA Drug Establishment
PALO ALTO, Calif., Jan. 26, 2022 /PRNewswire/ -- I Peace, Inc. (CEO: Koji Tanabe ), aPalo Alto-based biotech start-up, announced that its cell manufacturing facility is now listed on the FDA "Drug Establishments Current Registration Site." The registration recognizes I Peace's cell manufacturing f...
Adlai Nortye Announces Global License-out Agreement with Biotime for Several Products Including PD-L1 Inhibitor (AN4005) and Anti-hTNFR2 Antibody (AN3025)
NORTH BRUNSWICK, N.J. and HANGZHOU, China, Jan. 26, 2022 /PRNewswire/ -- Adlai Nortye Ltd. ("Adlai Nortye"), a clinical-stage biopharmaceutical company focused on the development of innovative cancer therapies, announced that it has entered into a Global License Agreement with Xiamen Biotime Biot...
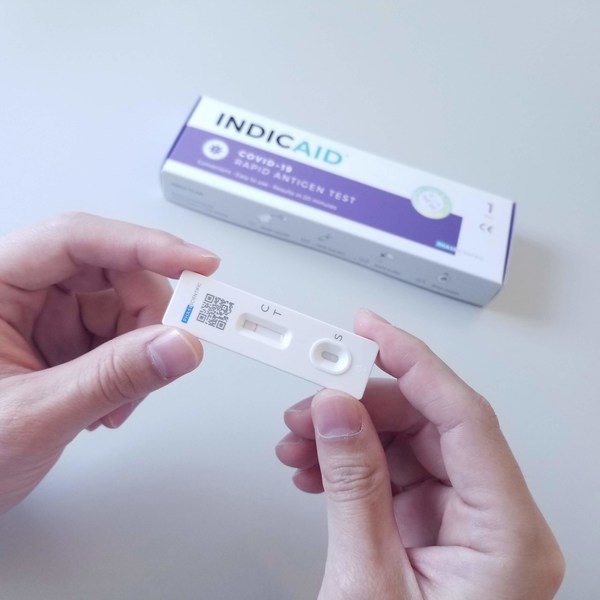
Singapore Grants Approval for INDICAID COVID-19 Rapid Antigen Test as Consumer Self-test for SARS-CoV-2 Control
HONG KONG and SINGAPORE, Jan. 26, 2022 /PRNewswire/ -- PHASE Scientific
International LTD
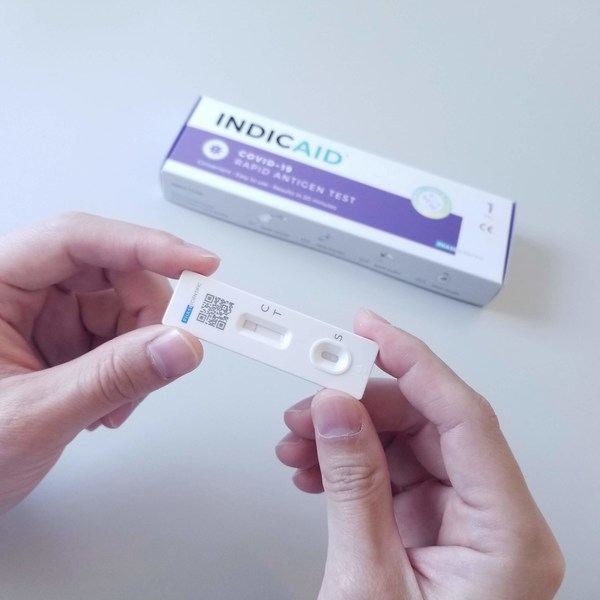
Singapore Grants Approval for INDICAID COVID-19 Rapid Antigen Test as Consumer Self-test for SARS-CoV-2 Control
HONG KONG, Jan. 26, 2022 /PRNewswire/ -- PHASE Scientific International LTD
RemeGen's Telitacicept Demonstrates Positive Results in Phase II Trial in Treatment of Primary Sjögren's Syndrome
YANTAI, China, Jan. 26, 2022 /PRNewswire/ -- RemeGen Co., Ltd. (9995.HK), a commercial-ready biotechnology company, recently announced positive results from the completion of its Phase II trial inChina for Telitacicept in the treatment of primary Sjögren's syndrome ("pSS"). A total of 42 patient...
GenScript ProBio Initiates a CDMO Contract For Next-Generation CD47 Antibody with InnoBation Bio
SEOUL, South Korea, Jan. 26, 2022 /PRNewswire/ -- GenScript ProBio, a global biopharmaceutical contract development and manufacturing organization (CDMO), announced onJanuary 26th, 2022 that it entered into a contract with InnoBation Bio to develop and manufacture clinical trial sample substances...
Hope Medicine Announces US FDA Clearance for Phase II Clinical Trial of A First-in-class Monoclonal Antibody, HMI-115, in Androgen Alopecia
SHANGHAI, Jan. 25, 2022 /PRNewswire/ -- Hope Medicine Inc. ('HopeMed'), a clinical stage innovative biopharmaceutical company, has recently announced that U.S. Food and Drug Administration (FDA) has approved its Investigational New Drug (IND) application for phase II study to evaluate HMI-115, a ...
Senhwa's Pidnarulex Receives US FDA Fast Track Designation for the Treatment of Solid Tumors with BRCA1/2, PALB2 and other HR Gene Mutations
TAIPEI and SAN DIEGO, Jan. 25, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced today that the US Food and Drug Administration (FDA) has granted Fast Track...
2022 Japan Prize Laureates Announced
TOKYO, Jan. 25, 2022 /PRNewswire/ -- The Japan Prize Foundation announced the winners of the 2022 Japan prize onJanuary 25. Prof. Katalin Kariko (Hungary/USA ) and Prof.Drew Weissman (USA) are co-winners of the Japan Prize in the field of "Materials and Production," and Prof.Christopher Field (USA...
Insilico Medicine Applauded by Frost & Sullivan for Enabling Rapid and Cost-Effective Drug Discovery and Development with Its Pharma.AI Platform
The Pharma.AI platform combining deep generative models (GAN), reinforcement learning (RL), transformers, and other modern machine learning techniques enables the identification of novel targets,generation of novel molecules non-existent in the chemical space, and prediction of outcomes for clini...
RemeGen's Telitacicept Demonstrates Positive Results in Phase II Trial in Treatment of Primary Sjögren's Syndrome
YANTAI, China, Jan. 24, 2022 /PRNewswire/ -- RemeGen Co., Ltd. (9995.HK), a commercial-ready biotechnology company, recently announced positive results from the completion of its Phase II trial inChina for Telitacicept in the treatment of primary Sjögren's syndrome ("pSS"). A total of 42 patient...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Announcement of "Cyber Sousa Award" Winners of the 16th Xiamen International Animation Festival
[Picked up by 272 media titles]
2024-11-26 16:48