Biotechnology
Regor Therapeutics Announces U.S. FDA Authorization to Conduct Regor's First-in-Human Clinical Trial with the Next Generation Targeted Inhibitor RGT-419B for Oncology
SHANGHAI, Dec. 27, 2021 /PRNewswire/ -- On December 23, Regor Therapeutics, a clinical-stage biotech company,announced authorization from the US Food and Drug Administration (FDA) to proceed with Regor's Phase 1 clinical development plans for RGT-419B. RGT-419B is a new generation CDK2/4/6, smal...
Laekna Presents Vision and Strategy at NewYorkBIO/NYSE Emerging Company Showcase
SHANGHAI and WARREN, N.J., Dec. 24, 2021 /PRNewswire/ -- Laekna was invited by NewYorkBIO and the New York Stock Exchange (NYSE) to present, as an emerging biotech company at the "Emerging Company Showcase" that was held earlier this month inNew York. Dr. Guy Rosenthal, Vice President, Head of C...
Convidecia™ Phase III Results Published in The Lancet
* 96.0% effective against severe disease 14 days post-vaccination for population aged 18 and above. * Single-dose vaccine received approvals in at least 10 markets including China, Mexico, Ecuador, Chile, Argentina, Hungary, Kirghizstan, Pakistan, Indonesia and Malaysia. TIANJIN, China, Dec. ...
Novavax and SK bioscience Expand Manufacturing Agreement
- Novavax secures additional manufacturing capacity through 2022 - SK bioscience secures long-term license to supply NVX-CoV2373 for the Korean market GAITHERSBURG, Md., Dec. 24, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializin...
Betagenon co-founder publishes that AMPK activator O304 prevents gene expression changes and remobilisation of histone marks in islets of diet-induced obese mice
STOCKHOLM, Dec. 23, 2021 /PRNewswire/ -- Betagenon AB, a Sweden-based company focused on development of AMPK activator compounds, today announced the publication by co-founderHelena Edlund of a new study demonstrating the prevention and reversal of gene expression and epigenetic changes to beta c...
Curocell, Breaks Ground on 'CAR-T Manufacturing GMP Facility'
DAEJEON, South Korea, Dec. 23, 2021 Curocell, currently on PhaseⅠCAR-T Therapy
clinical trial withCRC01 (anbalcabtagene autoleucel) has recently broken ground
for the new CAR-T Center in Dungok Residential & Industrial Area in Daejeon
International Science & Business Belt.
Luye Pharma Releases Top-Line Results from a Phase II Clinical Trial of Its New Antidepressant Ansofaxine Hydrochloride Extended-Release Tablets
CHONGQING, China, Dec. 23, 2021 /PRNewswire/ -- Luye Pharma Group today released the encouraging top-line results from a Phase II clinical trial of its new antidepressant, Ansofaxine Hydrochloride Extended-Release Tablets (LY03005), at the 19th National Psychiatry Conference of the Chinese Medica...
Lion TCR Receives FDA Fast Track Designation for its HBV-specific TCR T Cell Therapy for Hepatocellular Carcinoma
SINGAPORE and GUANGZHOU, China and LOS ANGELES, Dec. 23, 2021 /PRNewswire/ -- Lion TCR Pte Ltd today announced that it has received Fast Track Designation from United States Food and Drug Administration (U.S. FDA) for LioCyx-M004, autologous T-cells transfected with mRNA encoding Hepatitis B surf...
Aerogen Pharma Enters into Exclusive Agreements with Nuance Pharma to Advance Treatment of Respiratory Distress Syndrome in Premature Infants in China
SHANGHAI, Dec. 22, 2021 /PRNewswire/ -- Aerogen Pharma, a developer of innovative inhaled treatments for patients in critical care, and Nuance Pharma ("Nuance"), a specialty care focused biopharma with late-stage clinical programs and existing commercial operations, today announce an exclusive a...
Novavax Announces Initial Omicron Cross-Reactivity Data from COVID-19 Vaccine Booster and Adolescent Studies
- Two-dose primary regimen of NVX-CoV2373 demonstrated cross-reactive immune responses against Omicron (B.1.1.529) and other variants - Third dose produced increased immune responses comparable to or exceeding levels associated with protection in Phase 3 clinical trials, with a 9.3-fold IgG rise...
Origin Agritech Developing Nutrition Enhanced Corn Using CRISPR Gene Editing
BEIJING, Dec. 22, 2021 /PRNewswire/ -- Origin Agritech Ltd. (NASDAQ: SEED) (the "Company" or "Origin"), an agriculture technology company, announced today that it is developing nutritionally enhanced corn using CRISPR technology. Origin is currently in trials to develop new corn varieties that ha...
AMO Pharma Announces Expansion of Pivotal REACH-CDM Study in Congenital Myotonic Dystrophy
- Additional research centers now enrolling patients in Australia and New Zealand - Company has achieved 50 percent of target enrollment in global Phase 3 trial LONDON, Dec. 23, 2021 /PRNewswire/ -- AMO Pharma Limited ("AMO Pharma"), a privately held biopharmaceutical company focusing on r...
Angel Yeast Invests in New Enzyme Project Capable of 5,000 Tons Annual Production Output
YICHANG, China, Dec. 22, 2021 /PRNewswire/ -- Angel Enzyme Preparation
(Yichang) Co., Ltd., ("Angel Enzyme Preparation"), a wholly-owned subsidiary of
Angel Yeast Co., Ltd.
Exopharm Gains US Patent Enabling Commercial Production of Exosome Medicines
* US Patent and Trademark Office has granted Exopharm patent US 11202805, for its LEAP™ exosome purification technology. * LEAP™ enables large-scale, clinical grade commercial production of exosomes needed to underpin the emerging field of exosome medicines. * Exopharm is seeking potential p...
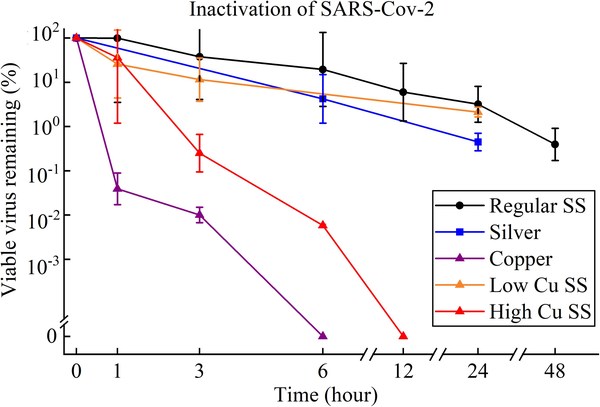
HKU teams develop the first anti-COVID-19 stainless steel
HONG KONG, Dec. 22, 2021 /PRNewswire/ -- The project team led by Professor Mingxin HUANG at the Department of Mechanical Engineering of the Faculty of Engineering of theUniversity of Hong Kong (HKU), in collaboration with Professor Leo Lit Man POON's research team at the Centre for Immunity and ...
World Health Organization SAGE Issues Interim Recommendations for Novavax COVID-19 Vaccine
- WHO Strategic Advisory Group of Experts on Immunization (SAGE) recommends primary two-dose vaccination series of NVX-CoV2373 in persons aged 18 and older - SAGE recommends additional third dose of NVX-CoV2373 administered to immunocompromised persons - Recommendation follows grant of WHO Emerg...
EVERSANA completes acquisition of Intouch Group, adds the premiere digital-first agency network to the market leader in next generation commercialization
CHICAGO and KANSAS CITY, Mo., Dec. 21, 2021 /PRNewswire/ -- EVERSANA™, the pioneer of next generation commercial services to the global life sciences industry, today announced it has completed its acquisition ofIntouch Group®, a full-service global agency network serving the pharmaceutical indust...
The New Era Biotechnology Brings The Cooperation Between China And Belarus To A New Level
MINSK, Belarus, Dec. 21, 2021 /PRNewswire/ -- New Era Biotechnology Co., Ltd is an enterprise fromthe Great Stone China Belarus Industrial Park (CBIP) in Minsk . It participated in the fourth edition of the China International Import Expo (CIIE) which opened inShanghai on November 5, 2021. Loc...
European Commission Grants Conditional Marketing Authorization for Novavax COVID-19 Vaccine
* Nuvaxovid™ COVID-19 Vaccine (recombinant, adjuvanted) is the first protein-based COVID-19 vaccine authorized for use inEurope * Novavax and the European Commission previously announced an advance purchase agreement for up to 200 million doses through 2023 * Authorization follows positive r...
Henlius' anti-PD-1 mAb MRCT achieved 15.38 months OS in first-line treatment of SCLC, reducing the risk of death by 38% of the overall population
* The ASTRUM-005 states that serplulimab combined with carboplatin-etoposide prolonged median OS in both the overall population and the Asian subgroup, the median overall survival (OS) in the serplulimab and placebo groups were 15.38 and 11.10 months, respectively, reducing risk of death by 38%...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00