Biotechnology
Over 68% of Farmers in SE Asia's Biggest Crop-Producing Countries Claim Climate Change as Key Challenge
SINGAPORE, Aug. 30, 2021 /PRNewswire/ -- A finding officially released today highlights a key challenge with regional food production – chiefly that a significant number of growers inSoutheast Asia's largest agricultural-producing countries areconcerned with the impact of climate change (68.5%). ...
GeneQuantum announces Dr. Yi Xia as Senior Vice President of Statistics and Data Science
SUZHOU, China, Aug. 29, 2021 /PRNewswire/ -- GeneQuantum Healthcare, an industry leading biopharmaceutical company dedicated to next generation bioconjugate drugs through innovative intelligent ligase-dependent conjugation (iLDC) technology platform, announces the appointment of Dr.Yi Xia as Seni...
Triad Life Sciences completes A$25m funding round, anticipates ASX listing in coming months
MEMPHIS, Tenn., Aug. 29, 2021 /PRNewswire/ -- Innovative US based biotech companyTriad Life Sciences, Inc (Triad or the Company), is pleased to announce the completion of a successfulA$25 million funding round which saw strong support from major Australian institutional investors. The convertible...
Kenya Grants Emergency Use Authorization for the INDICAID(TM) COVID-19 Rapid Antigen Test
HONG KONG, Aug. 30, 2021 /PRNewswire/ -- PHASE Scientific announces that its INDICAID™ COVID-19 Rapid Antigen Test (INDICAID™) has received Emergency Use Authorization from the Ministry of Health (MOH) of the Republic ofKenya on 17 August 2021. The authorization enables the test to be used in Ken...
Innovent to Present New Data on Sintilimab and Other Molecules at the European Society for Medical Oncology (ESMO) Congress 2021
SAN FRANCISCO and SUZHOU, China, Aug. 30, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, metabolic, autoimmune, ophthalmology and...
Hope Medicine Inc. Announced Dr. Henri Nico Doods as Chief Executive Officer
SHANGHAI, Aug. 30, 2021 /PRNewswire/ -- Hope Medicine Inc. ('HopeMed'), a clinical stage innovative biopharmaceutical company, has recently announced that the former President and Head of Research & Clinical Development, Dr.Henri Nico Doods, will serve as the company's Chief Executive Officer. Dr...
InnoCare Releases 2021 Half Year Results and Business Highlights
BEIJING, Aug. 29, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969), a leading biopharmaceutical company focusing on cancer and autoimmune diseases, today announced 2021 half year results which ended onJune 30, 2021. Dr. Jasmine Cui, Co-founder, Chairwoman and CEO of InnoCare, said, "On the fo...
JNCCN Study Reveals Neuroendocrine Tumor Mortality Patterns to Inform Treatment Decisions
Population–based cohort study from Canada is first to describe patterns of mortality, both cancer-related and otherwise, for people with different types of NETs. PLYMOUTH MEETING, Pa., Aug. 27, 2021 /PRNewswire/ -- Among all patients with neuroendocrine tumors (NETs), the risk of dying of cancer...
Insilico Medicine and 4B Technologies Announce Strategic Collaboration in Advancing Novel Drug Discovery for Neurodegenerative Diseases
SHANGHAI, Aug. 27, 2021 /PRNewswire/ -- Insilico Medicine ("Insilico"), an end-to-end artificial intelligence (AI)-driven drug discovery company, and4B Technologies Co., Ltd. ("4B Technologies"), a leading end-to-end innovative biopharmaceutical company focusing on nervous system diseases, signed...
3SBio announces 2021 interim results, with strong growth in core businesses and significant progress in clinical development
HONG KONG, Aug. 26, 2021 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today unveiled its 2021 interim results. 3SBio maintained strong performance in core businesses, with core products occupying a leading market share. Meanwhile, the Company continuously increased ...
Everest Medicines Announces First Person Dosed in Global Phase 3 Registration Trial of Sacituzumab Govitecan-Hziy in China for Metastatic Urothelial Cancer
SHANGHAI, Aug. 26, 2021 /PRNewswire/ -- Everest Medicines
INOVIO Receives Authorization to Conduct Phase 3 Efficacy Trial of its COVID-19 DNA Vaccine Candidate, INO-4800
INOVIO's global Phase 3 efficacy trial receives authorization to proceed from Brazil; other countries to follow INOVIO to conduct INNOVATE Phase 3 efficacy trial with partner Advaccine in areas of world in need of COVID-19 vaccines PLYMOUTH MEETING, Pa., Aug. 26, 2021 /PRNewswire/ -- INOVIO (NAS...
RedHill Biopharma Reports Second Quarter 2021 Financial Results and Operational Highlights
TEL AVIV, Israel and RALEIGH, N.C., Aug. 26, 2021 /PRNewswire/ -- RedHill
Biopharma Ltd.
RedHill Biopharma's Opaganib Demonstrates Strong Inhibition of COVID-19 Delta Variant
TEL AVIV, Israel and RALEIGH, N.C., Aug. 26, 2021 /PRNewswire/ -- RedHill
Biopharma Ltd.
Sai Life Sciences becomes a signatory of the United Nations Global Compact (UNGC)
HYDERABAD, India, Aug. 26, 2021 /PRNewswire/ -- Sai Life Sciences, a leading
globalContract Research, Development & Manufacturing Organization
XABT Donates 2019-nCoV Nucleic Acid Detection Kits to Vientiane, Laos
BEIJING, Aug. 25, 2021 /PRNewswire/ -- Atsaphangthong Siphandone, Mayor of Vientiane, the capital of Laos, recently issued an honorary certificate to Beijing Applied Biological Technologies (XABT) for the donation of 2019-nCoV Nucleic Acid Detection Kits to supportVientiane's efforts at epidemic ...
Innovent Announced 2021 Interim Results with Total Revenue of RMB 1,941.8 Million and Accelerated Global R&D Footprint
SAN FRANCISCO and SUZHOU, China, Aug. 25, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Tigermed Reports 2021 Interim Results with Strong Financial Growth
HANGZHOU, China, Aug. 25, 2021 /PRNewswire/ -- Hangzhou Tigermed Consulting Co., Ltd. (stock code: 300347.SZ / 3347.HK), a leading provider of innovative clinical research solutions for biopharmaceutical and medical device industry, reports its interim results for the six months endedJune 30, 202...
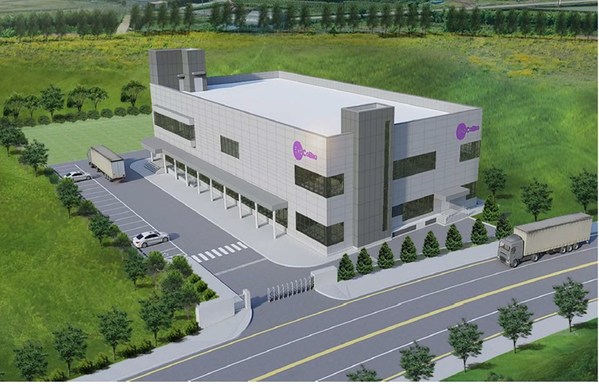
ExoCoBio to Build the World's First Clinical Grade Exosome GMP Manufacturing Facility
* $20 million investment for a new Mfg & R&D facility * Strategic platform for partnering & CDMO business SEOUL, South Korea, Aug. 25, 2021 /PRNewswire/ -- ExoCoBio Inc., the 4th largest exosome player in the world, announced the initiation of its "Project EGMP", starting with the constructio...
Angel Yeast Announces Acquisition of Bio Sunkeen's Yeast Relevant Assets
YICHANG, China, Aug. 25, 2021 /PRNewswire/ -- Angel Yeast Co., Ltd ("Angel Yeast
")(en.angelyeast.com
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00