Biotechnology
FDA Accepts Filing of New Drug Application for Tepotinib for the Treatment of Patients with Metastatic NSCLC with METex14 Skipping Alterations
- Tepotinib granted Priority Review and is being evaluated under FDA Real-Time Oncology Review (RTOR) pilot program - Tepotinib is a highly targeted inhibitor of c-MET that is administered as a once-daily oral tablet - Data show robust, consistent and durable clinical response across different ...
Global Cord Blood Corporation Reports Financial Results for the First Quarter of Fiscal 2021
HONG KONG, Aug. 25, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) ("GCBC" or the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced its unaudited financial results for...
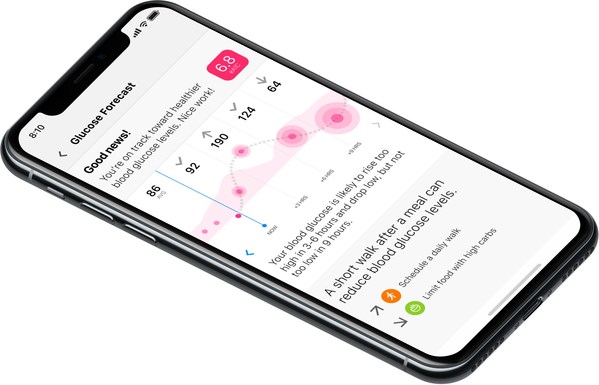
One Drop Announces Nearly $100M Financing And Commitments By Bayer
$98.7M financing, development fees and commercial milestone commitments accelerate the development and commercialization of One Drop's digital health platform and data-driven predictive capabilities across multiple therapeutic areas NEW YORK, Aug. 24, 2020 /PRNewswire/ -- One Drop, a leader in d...
Singapore's Restalyst develops highly sensitive COVID-19 antibody test comparable to market leaders
SINGAPORE, Aug. 24, 2020 /PRNewswire/ -- Singapore biomedical firm Restalyst has developed a COVID-19 antibody test, COVID19N-REAAD™, which will be instrumental in establishing disease prevalence in populations and the exposure risk factors associated with infection. This will enable healthcare a...
US FDA Awards Orphan Drug Designation (ODD) To Paxalisib For Malignant Glioma, Including DIPG
SYDNEY, Aug. 24, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that the United States Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to Kazia's paxalisib (formerl...
Cipla and Stempeutics collaborate for launch of Stempeucel®, first 'Made in India' Cell Therapy to treat Critical Limb Ischemia (CLI)
MUMBAI, India, Aug. 21, 2020 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its partner Stempeutics Research Pvt. Ltd has received regulatory approval by the Drug Controller General ofIndia (DCGI) for the launch of Stempeucel® in India. The...
Ascletis Completed Bridging Study of ASC18, a One-pill, Once-a-day Complete HCV Oral Regimen
HANGZHOU and SHAOXING, China, Aug. 20, 2020 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX code: 1672) announces today that it completed bridging study of ASC18, first one-pill, once-a-day fixed dose combination (FDC) as the complete hepatitis C treatment developed by a Chinese biotech. ASC18 FDC co...
Low-Cost Nafamostat Blocks SARS-CoV-2 Infection in Human Lung Cells, Preclinical Study Finds
SAN DIEGO, Aug. 20, 2020 /PRNewswire/ -- A preclinical study testing the low-cost generic drug nafamostat in human cells shows it can help block SARS-CoV-2 infection, raising excitement around Covistat's work to develop prophylactic and therapeutic COVID-19 treatments. Biopharmaceutical company ...
CARsgen Therapeutics Receives IND Clearance from the NMPA for CT041 CLDN18.2-CAR-T Cells
SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- CARsgen Therapeutics Co., Ltd., a clinical-stage biopharmaceutical company, today announced that the National Medical Products Administration (NMPA) has cleared its Investigational New Drug (IND) application for the first-in-class drug candidate CT041, auto...
Visiopharm granted patents for stain quality management in Tissue Biomarkers
HOERSHOLM, Denmark, Aug. 20, 2020 /PRNewswire/ -- Visiopharm®, the Denmark -based leader in artificial intelligence-driven image analysis, tissue mining, and precision pathology, has been granted patents in the US andEurope for an AI and image analysis-based approach to quantifying staining qualit...
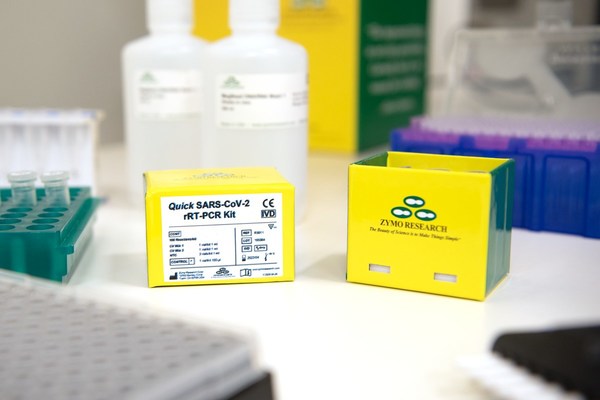
Zymo Research Granted CE IVD Mark for Quick SARS-CoV-2 rRT-PCR Kit
IRVINE, California, Aug. 20, 2020 /PRNewswire/ -- Zymo Research announced that it was recently granted the CE IVD Mark for itsQuick SARS-CoV-2 rRT-PCR Kit. To obtain this certification the product had to comply with the Directive 98/79/EC of the European Parliament and of the Council of27 October...
US FDA Awards Fast Track Designation (FTD) to Paxalisib for Glioblastoma
SYDNEY, Aug. 20, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that the United States Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) to Kazia's paxalisib (forme...
Harbour BioMed, Utrecht University, Erasmus Medical Center, Viroclinics-DDL and Kiadis Pharma Announce Collaboration to Develop Monoclonal Antibody, Natural Killer Cell Combination Approach to Treating COVID-19
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Aug. 19, 2020 /PRNewswire/ -- Harbour BioMed (HBM), and its partnersUtrecht University and Erasmus Medical Center, today announced a new research collaboration with Viroclinics-DDL and Kiadis Pharma (Euronext Amsterdam and Brussels: K...
Global Entertainment Giant YOOZOO Aligns with Health Tech Company Plano to Tackle the Global Myopia Epidemic
SINGAPORE, Aug. 19, 2020 /PRNewswire/ -- Plano Pte Ltd (hereinafter, Plano), a Singapore-based health technology startup, and one of the world's leading providers of interactive entertainment, YOOZOO, have inked a Memorandum of Understanding to address the global burden of myopia inSingapore, the...
CStone Pharmaceuticals Reports Financial Results for the First Half of 2020
SUZHOU, China, Aug. 19, 2020 /PRNewswire/ -- CStone Pharmaceuticals ("CStone"; HKEX: 2616), a leading biopharmaceutical company focused on developing and commercializing innovative immuno-oncology (IO) therapies and precision medicines, today reported recent business highlights and financial resu...
PharmAbcine announces the execution of a Material Cooperative Research and Development Agreement (MCRADA) with the NIH to evaluate the efficacy of PMC-403 to treat SCLS
DAEJEON, South Korea, Aug. 18, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) entered into a Materials Cooperative Research and Development Agreement (MCRADA) with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), in whic...
Ascentage Pharma Releases 2020 Interim Results, Reporting the Company's First New Drug Application and Advances in Global Strategic Collaborations
SUZHOU, China and ROCKVILLE, Md., Aug. 18, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced its interim results for the ...
Global Cord Blood Corporation to Report First Quarter Fiscal 2021 Financial Results
HONG KONG, Aug. 18, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) (the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that it plans to release financial results fo...
Global Cord Blood Corporation to Report First Quarter Fiscal 2021 Financial Results
HONG KONG, Aug. 18, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) (the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that it plans to release financial results fo...
China Biologic Reports Financial Results for the Second Quarter of 2020
BEIJING, Aug. 17, 2020 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today announced its unaudited financial results for the second quarter of 2020. Second Quart...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00