Biotechnology
Transcenta Presents First Data from Phase 1 Study of pH-Dependent PDL1 Antibody MSB2311 in Patients with Pre-treated Advanced Solid Tumors and Select Hematological Malignancies
SUZHOU, China, May 27, 2020 /PRNewswire/ -- Transcenta Holding Limited ("Transcenta"), a global biotherapeutics company with fully-integrated capabilities in discovery,development and manufacturing of antibody-based therapeutics, announced today results reported for the first time from a Phase 1...
Ajinomoto Bio-Pharma Services Signs Manufacturing Agreement with Humanigen for Lenzilumab, Currently in FDA-Approved Phase III Study for COVID-19
SAN DIEGO, May 27, 2020 /PRNewswire/ -- Ajinomoto Bio-Pharma Services ("Aji Bio-Pharma"), a leading provider of biopharmaceutical contract development and manufacturing services, is pleased to announce it has entered into a manufacturing agreement with Humanigen, Inc., for the fill finish supply ...
Jennewein Publishes New Results on the Norovirus-inhibiting Effect of Complex Oligosaccharides
RHEINBREITBACH, Germany, May 27, 2020 /PRNewswire/ -- Jennewein Biotechnologie GmbH today announces the publication of the latest results from its norovirus research program in theJournal of Biotechnology. Breast-fed infants are less likely than bottle-fed infants to contract norovirus infecti...
Daewoong Pharmaceutical Acquires Halal Certification for 'Easyef' through Daewoong Infion
SEOUL, South Korea and JAKARTA, Indonesia, May 27, 2020 /PRNewswire/ -- Daewoong Pharmaceutical (CEOSengho Jeon) announced on May 25 that the Indonesian joint venture Daewoong Infion (CEOChang Woo Suh) obtained Halal certification for the diabetic foot ulcer drug 'Easyef topical solution(Easyef)...
Innovent Announces First Patient Dosed in A Phase 1 Clinical Trial of Anti-TIGIT Monoclonal Antibody in China
SUZHOU, China, May 26, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic and other major diseases, today anno...
Tavotek Announces a Research Agreement with Genmab for the Development of Bispecific Antibodies using Genmab's DuoBody® Platform against a Pair of Disease Relevant Targets
AMBLER, Pa., May 25, 2020 /PRNewswire/ -- Tavotek Biotherapeutics, a biotech company which develops novel biologics for autoimmune diseases and oncology, announced that it has entered into a research agreement with Genmab A/S of Denmark to create and develop bispecific antibodies using Genmab's Du...
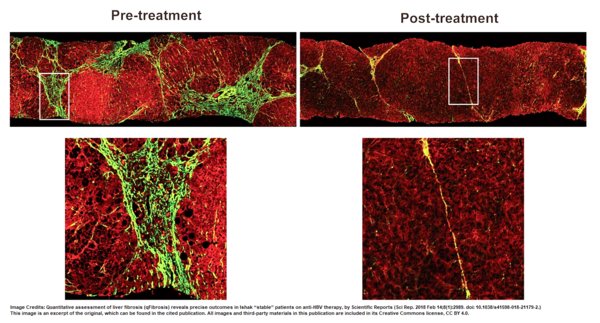
HistoIndex's AI-based Digital Pathology Platform: A Validated Quantification Tool Recommended in the Guidelines for the Prevention and Treatment of Chronic Hepatitis B in China
SINGAPORE, May 25, 2020 /PRNewswire/ -- Singapore medtech company HistoIndex
GSK partners with Samsung Biologics to secure additional manufacturing capacity for innovative biopharmaceutical portfolio
LONDON and SONGDO, South Korea, May 21, 2020 /PRNewswire/ -- GlaxoSmithKline plc (LSE/NYSE: GSK) and Samsung Biologics (207940.KS) have entered into a partnership to provide GSK with additional capacity to manufacture and supply GSK's innovative biopharmaceutical therapies. Under the terms of th...
Transcenta Announces to Present Preclinical Data of TST001 at 2020 AACR Virtual Annual Meeting II
SUZHOU, China, May 21, 2020 /PRNewswire/ -- Transcenta, a global biotherapeutics company that fully integrates antibody-based biotherapeutics discovery, development and manufacturing, today announced that it will present preclinical data of TST001, a humanized Claudin 18.2 (CLDN18.2) monoclonal ...
Takara Bio Selects AGC Biologics as Manufacturer of Plasmid DNA Intermediate for a Vaccine Against COVID-19 (SARS-CoV-2)
SEATTLE, May 21, 2020 /PRNewswire/ -- AGC Biologics, a global Contract Development and Manufacturing Organization (CDMO) for Biopharmaceuticals, has announced its partnership withTakara Bio.The companies will partner in the fight against COVID-19 by collaborating on a prophylaxis DNA vaccine, wit...
Catalent Announces Acquisition of Japanese Facility to Provide Local and Global Clinical Supply Solutions
SOMERSET, N.J., May 21, 2020 /PRNewswire/ -- Catalent (NYSE:CTLT), a global leader in clinical supply services, today announced a deal to acquire a clinical packaging facility in Minakuchi, located in the Shiga prefecture of Japan, from Teva-Takeda Pharmaceuticals, Nagoya Aichi, Japan. This purcha...
China Biologic Comments on the Xinjiang Deyuan and Shuanglin Transaction
BEIJING, May 20, 2020 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today declared that Xinjiang Deyuan Bioengineering Co., Ltd. ("Xinjiang Deyuan"), China Biolog...
China Biologic Reports Financial Results for the First Quarter of 2020
BEIJING, May 20, 2020 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO, "China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today announced its unaudited financial results for the first quarter of 2020. First Quarter ...
VeChain and I-Dante Co-developed E-HCert, A Blockchain-based COVID-19 Records App For the Citizens of Cyprus
SHANGHAI, May 20, 2020 /PRNewswire/ -- With the pandemic putting healthcare systems under strain worldwide, Electronic Health Record (EHR) systems are also shifting requirements for data security and sharing. As the leading public blockchain platform, VeChain furthers the collaboration with I-Dan...
VolitionRx Announces Pricing of Underwritten Public Offering of Common Stock
AUSTIN, Texas, May 20, 2020 /PRNewswire/ -- VolitionRx Limited
QPS Continues to Expand UPLC-HRMS Quantitation Capabilities to Support Gene Therapy and Protein Drug Development
NEWARK, Delaware, May 19, 2020 /PRNewswire/ -- QPS, a global contract research organization (CRO) that provides discovery, preclinical, and clinical drug development services, is reinforcing its focus on qualitative and quantitative bioanalysis of biotherapeutics. QPS announces an expanded and up...
Invivoscribe Announces FDA Approval for Distribution of the LeukoStrat CDx FLT3 Mutation Assay as an IVD Kit in the United States
SAN DIEGO, May 19, 2020 /PRNewswire/ -- Invivoscribe to offer the LeukoStrat® CDxFLT3 Mutation Assay as an FDA approved kit with analysis software. In 2017, Invivoscribe's LeukoStrat® CDx FLT3 Mutation Assay became the first FDA approvedFLT3 test and launched as a testing service at LabPMM, Invi...
Excelra to Provide Lawrence Livermore National Laboratory With Small Molecule Medicinal Chemistry Intelligence Data to Help Develop Drug Design Platform
HYDERABAD, India, May 19, 2020 /PRNewswire/ -- Under a three-year agreement,
Excelra
Exyte and Univercells Technologies combine forces for rapid deployment of vaccine production plants in the wake of the COVID-19 pandemic
* Exyte offers the new ExyCell® modular technology for fast-track construction of biologicals production facilities compliant with the current good manufacturing practice (cGMP). * Univercells Technologies delivers the integrated NevoLine™ biomanufacturing platform, including scale-X™ bioreac...
WuXi Biologics Closes Land Deal to Build State-of-the-Art Biologics Production Facility in the United States
SHANGHAI and WORCESTER, Mass., May 19, 2020 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, and the Worcester Business Development Corporation (WBDC) announced today the successful signing of a land deal for WuXi Bio...
Week's Top Stories
Most Reposted
ASTON MARTIN ARAMCO FORMULA ONE® TEAM ANNOUNCE PEPPERSTONE AS OFFICAL TRADING PARTNER
[Picked up by 308 media titles]
2025-01-07 09:00Brewing Connections and Spotlighting Café Culture: MIFB 2025 Hosts MNCC in Collaboration with MSCA
[Picked up by 307 media titles]
2025-01-06 14:00RuggON Unveils VORTEX Vehicle Mount Computer with AI-Enhanced, SATCOM-Ready
[Picked up by 306 media titles]
2025-01-06 21:00iWOW Unveils Age-Tech Innovations at CES 2025, Expanding Portfolio of IoT Solutions for Ageing-In-Place
[Picked up by 294 media titles]
2025-01-07 21:18CELEBRATE THE LUNAR NEW YEAR 2025 AT HOIANA RESORT & GOLF, WORLD'S LEADING FULLY INTEGRATED RESORT
[Picked up by 292 media titles]
2025-01-06 19:25