Health Care/Hospital
US FDA Awards Orphan Drug Designation (ODD) To Paxalisib For Malignant Glioma, Including DIPG
SYDNEY, Aug. 24, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that the United States Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to Kazia's paxalisib (formerl...
Harbour BioMed Presented Its Newly Discovered BCMA x CD3 Bispecific Antibody at Cell Engager Summit 2020
CAMBRIDGE, Mass., ROTTERDAM, Netherlands, and SUZHOU, China, Aug. 21, 2020 /PRNewswire/ -- Harbour BioMed (HBM), a global, clinical-stage, innovative biopharmaceutical company, presented its newly discovered Bispecific Antibody - BCMA x CD3 (HBM7020) at the Cell engager Summit. HBM7020 was develo...
Zhongchao Inc. Offers Advanced Workshops in Partnership with European Wound Management Association (EWMA)
SHANGHAI, Aug. 21, 2020 /PRNewswire/ -- Zhongchao Inc. (NASDAQ: ZCMD) ("Zhongchao" or the "Company"), a healthcare services company offering patient management, online healthcare information, professional training and educational services, today announced that it continues to partner with Europe...
Ping An Healthcare and Technology Company Limited Announces 2020 Interim Report
SHANGHAI, Aug. 21, 2020 /PRNewswire/ -- Ping An Good Doctor ("Ping An Good Doctor", 01833.HK), the world's leading online healthcare platform, announced the revenue ofRMB 2.747 billion for the first half of this year, according to the company's latest financial report. Ping An Good Doctor perf...
KPMG and DXY launch book titled "Human: Solving the Global Workforce Crisis in Healthcare"
HONG KONG, Aug. 21, 2020 /PRNewswire/ -- KPMG and Dingxiangyuan (DXY), an online networking site for healthcare professionals, jointly hosted a virtual conference for the launch of the Chinese version of the book,Human: Solving the Global Workforce Crisis in Healthcare. Attendees included Mark ...
Cipla and Stempeutics collaborate for launch of Stempeucel®, first 'Made in India' Cell Therapy to treat Critical Limb Ischemia (CLI)
MUMBAI, India, Aug. 21, 2020 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its partner Stempeutics Research Pvt. Ltd has received regulatory approval by the Drug Controller General ofIndia (DCGI) for the launch of Stempeucel® in India. The...
Ping An Healthcare and Technology Company Limited reports revenue of RMB 2.747 billion for H1 2020
SHANGHAI, Aug. 21, 2020 /PRNewswire/ -- Ping An Good Doctor ("Ping An Good
Doctor", 01833.HK), the world's leading online healthcare platform, announced
the revenue ofRMB 2.747 billion for the first half of this year, according to
the company's latest financial report.
Clarivate Launches Coronavirus, Virology, and Infectious Disease (CVID) Data Lake to Accelerate Research, Preparedness and Response to Future Pandemics
Broad and deep intelligence -- harmonized and accessed via one single, secure source -- powers unique insights not possible via disconnected, siloed datasets LONDON, Aug. 20, 2020 /PRNewswire/ -- Clarivate Plc (NYSE: CCC), a global leader in providing trusted information and insights to accelerat...
South Korea's KT Develops AI-Based COVID-19 Research
SEOUL, South Korea, Aug. 20, 2020 /PRNewswire/ -- KT Corp. (KRX: 030200; NYSE: KT),South Korea's largest telecommunications company, is developing a COVID-19 infection risk assessment model, using its artificial intelligence (AI) and Big Data-based digital contact tracing technology, adding to gl...
Hong Kong's Hang Fook Elderly Home Adopts iBonus(R) Digital Prevention System Aimed at Helping Guard Against COVID-19
HONG KONG, Aug. 20, 2020 /PRNewswire/ -- One of the key reasons that COVID-19 has caused a great concern is its long incubation period. Most experts agree that for up to 14 days many affected patients have no obvious symptoms. Since nobody knows where these people have been or who they have been ...
Ascletis Completed Bridging Study of ASC18, a One-pill, Once-a-day Complete HCV Oral Regimen
HANGZHOU and SHAOXING, China, Aug. 20, 2020 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX code: 1672) announces today that it completed bridging study of ASC18, first one-pill, once-a-day fixed dose combination (FDC) as the complete hepatitis C treatment developed by a Chinese biotech. ASC18 FDC co...
Sihuan Pharmaceutical Introduced Investors for Capital Increase to Accelerate Solid-liquid Double Chamber Infusion Soft Bag Industry
HONG KONG, Aug. 20, 2020 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. (HKEX: 0460 "Sihuan Pharmaceutical" or the "Company", together with its subsidiaries, the "Group") is pleased to announce that Beijing Ruiye Drugs Manufacture Co., Ltd. ("Beijing Ruiye"), an associate of the Grou...
Low-Cost Nafamostat Blocks SARS-CoV-2 Infection in Human Lung Cells, Preclinical Study Finds
SAN DIEGO, Aug. 20, 2020 /PRNewswire/ -- A preclinical study testing the low-cost generic drug nafamostat in human cells shows it can help block SARS-CoV-2 infection, raising excitement around Covistat's work to develop prophylactic and therapeutic COVID-19 treatments. Biopharmaceutical company ...
CARsgen Therapeutics Receives IND Clearance from the NMPA for CT041 CLDN18.2-CAR-T Cells
SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- CARsgen Therapeutics Co., Ltd., a clinical-stage biopharmaceutical company, today announced that the National Medical Products Administration (NMPA) has cleared its Investigational New Drug (IND) application for the first-in-class drug candidate CT041, auto...
LumiraDx Receives FDA Emergency Use Authorization for Point of Care COVID-19 Antigen Test
LONDON, Aug. 20, 2020 /PRNewswire/ -- LumiraDx, the next-generation point of care diagnostic company, announced today that it hasreceived Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the LumiraDx SARS-CoV-2 antigen test, which will help meet the global ch...
111, Inc. Announces Second Quarter 2020 Unaudited Financial Results
SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a company dedicated to digitally connecting patients with drugs and healthcare services inChina, today announced its unaudited financial results for the second quarter endedJune 30, 2020. Second Quarter 2020...
111, Inc. Announces New Capital Injection and Strategic Plan to Expand Access to China's Capital Markets
* Completion of new capital injection in principal subsidiary Yao Fang Shanghai * Initiation to list shares of principal subsidiary Yao Fang Shanghai on the STAR Market * 111 firmly committed to maintaining Nasdaq listing status SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- 111, Inc. ("111" or th...
Zepp Unveils Zepp E, A New Stylish Line-Up of Wearables Designed with Wellness in Mind
SHENZHEN, China, Aug. 20, 2020 /PRNewswire/ -- Zepp, a professional-grade brand for the digital management of users' wellbeing, today unveiled a refreshed line-up of wearables, kicking off with the Zepp E series. With wellbeing at the top of everyone's agenda in 2020, the all-new Zepp E series ha...
Visiopharm granted patents for stain quality management in Tissue Biomarkers
HOERSHOLM, Denmark, Aug. 20, 2020 /PRNewswire/ -- Visiopharm®, the Denmark -based leader in artificial intelligence-driven image analysis, tissue mining, and precision pathology, has been granted patents in the US andEurope for an AI and image analysis-based approach to quantifying staining qualit...
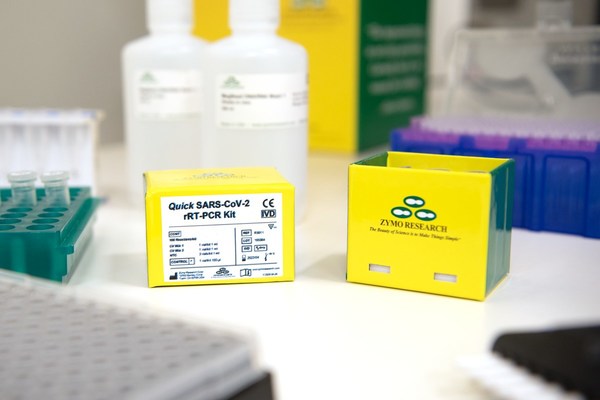
Zymo Research Granted CE IVD Mark for Quick SARS-CoV-2 rRT-PCR Kit
IRVINE, California, Aug. 20, 2020 /PRNewswire/ -- Zymo Research announced that it was recently granted the CE IVD Mark for itsQuick SARS-CoV-2 rRT-PCR Kit. To obtain this certification the product had to comply with the Directive 98/79/EC of the European Parliament and of the Council of27 October...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00