Medical/Pharmaceuticals
Adlai Nortye Announces First Patient Dosed in Phase 1b Clinical Trial of AN0025 (EP4 Antagonist) in Combination with Merck's KEYTRUDA® (pembrolizumab) for Advanced Solid Tumors
NORTH BRUNSWICK, N.J., Aug. 24, 2020 /PRNewswire/ -- Adlai Nortye Ltd. ("Adlai Nortye"), a global clinical-stage biopharmaceutical company today announced that the first patient has been dosed in a phase1b clinical trial (AN0025S0103) to evaluate AN0025, an investigational, potentially first in c...
SDIC leads the investment of Xuanzhu Biopharmaceutical with RMB 800 million, promoting innovation and R&D
HONG KONG, Aug. 24, 2020 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. (HKEX: 0460 "Sihuan Pharmaceutical" or the "Company", together with its subsidiaries, the "Group") is pleased to announce that Xuanzhu Biopharmaceutical, its innovative drug research and development platform, su...
US FDA Awards Orphan Drug Designation (ODD) To Paxalisib For Malignant Glioma, Including DIPG
SYDNEY, Aug. 24, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that the United States Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to Kazia's paxalisib (formerl...
Harbour BioMed Presented Its Newly Discovered BCMA x CD3 Bispecific Antibody at Cell Engager Summit 2020
CAMBRIDGE, Mass., ROTTERDAM, Netherlands, and SUZHOU, China, Aug. 21, 2020 /PRNewswire/ -- Harbour BioMed (HBM), a global, clinical-stage, innovative biopharmaceutical company, presented its newly discovered Bispecific Antibody - BCMA x CD3 (HBM7020) at the Cell engager Summit. HBM7020 was develo...
Cipla and Stempeutics collaborate for launch of Stempeucel®, first 'Made in India' Cell Therapy to treat Critical Limb Ischemia (CLI)
MUMBAI, India, Aug. 21, 2020 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its partner Stempeutics Research Pvt. Ltd has received regulatory approval by the Drug Controller General ofIndia (DCGI) for the launch of Stempeucel® in India. The...
Ascletis Completed Bridging Study of ASC18, a One-pill, Once-a-day Complete HCV Oral Regimen
HANGZHOU and SHAOXING, China, Aug. 20, 2020 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX code: 1672) announces today that it completed bridging study of ASC18, first one-pill, once-a-day fixed dose combination (FDC) as the complete hepatitis C treatment developed by a Chinese biotech. ASC18 FDC co...
Sihuan Pharmaceutical Introduced Investors for Capital Increase to Accelerate Solid-liquid Double Chamber Infusion Soft Bag Industry
HONG KONG, Aug. 20, 2020 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. (HKEX: 0460 "Sihuan Pharmaceutical" or the "Company", together with its subsidiaries, the "Group") is pleased to announce that Beijing Ruiye Drugs Manufacture Co., Ltd. ("Beijing Ruiye"), an associate of the Grou...
Low-Cost Nafamostat Blocks SARS-CoV-2 Infection in Human Lung Cells, Preclinical Study Finds
SAN DIEGO, Aug. 20, 2020 /PRNewswire/ -- A preclinical study testing the low-cost generic drug nafamostat in human cells shows it can help block SARS-CoV-2 infection, raising excitement around Covistat's work to develop prophylactic and therapeutic COVID-19 treatments. Biopharmaceutical company ...
CARsgen Therapeutics Receives IND Clearance from the NMPA for CT041 CLDN18.2-CAR-T Cells
SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- CARsgen Therapeutics Co., Ltd., a clinical-stage biopharmaceutical company, today announced that the National Medical Products Administration (NMPA) has cleared its Investigational New Drug (IND) application for the first-in-class drug candidate CT041, auto...
LumiraDx Receives FDA Emergency Use Authorization for Point of Care COVID-19 Antigen Test
LONDON, Aug. 20, 2020 /PRNewswire/ -- LumiraDx, the next-generation point of care diagnostic company, announced today that it hasreceived Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the LumiraDx SARS-CoV-2 antigen test, which will help meet the global ch...
111, Inc. Announces Second Quarter 2020 Unaudited Financial Results
SHANGHAI, Aug. 20, 2020 /PRNewswire/ -- 111, Inc. ("111" or the "Company") (NASDAQ: YI), a company dedicated to digitally connecting patients with drugs and healthcare services inChina, today announced its unaudited financial results for the second quarter endedJune 30, 2020. Second Quarter 2020...
Visiopharm granted patents for stain quality management in Tissue Biomarkers
HOERSHOLM, Denmark, Aug. 20, 2020 /PRNewswire/ -- Visiopharm®, the Denmark -based leader in artificial intelligence-driven image analysis, tissue mining, and precision pathology, has been granted patents in the US andEurope for an AI and image analysis-based approach to quantifying staining qualit...
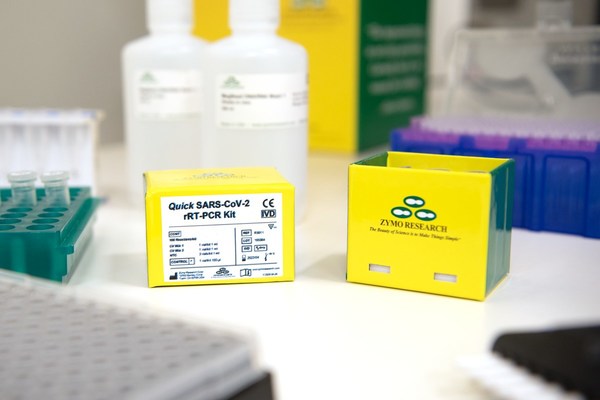
Zymo Research Granted CE IVD Mark for Quick SARS-CoV-2 rRT-PCR Kit
IRVINE, California, Aug. 20, 2020 /PRNewswire/ -- Zymo Research announced that it was recently granted the CE IVD Mark for itsQuick SARS-CoV-2 rRT-PCR Kit. To obtain this certification the product had to comply with the Directive 98/79/EC of the European Parliament and of the Council of27 October...
US FDA Awards Fast Track Designation (FTD) to Paxalisib for Glioblastoma
SYDNEY, Aug. 20, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that the United States Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) to Kazia's paxalisib (forme...
Harbour BioMed, Utrecht University, Erasmus Medical Center, Viroclinics-DDL and Kiadis Pharma Announce Collaboration to Develop Monoclonal Antibody, Natural Killer Cell Combination Approach to Treating COVID-19
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Aug. 19, 2020 /PRNewswire/ -- Harbour BioMed (HBM), and its partnersUtrecht University and Erasmus Medical Center, today announced a new research collaboration with Viroclinics-DDL and Kiadis Pharma (Euronext Amsterdam and Brussels: K...
Jamjoom Pharmaceuticals Earns Acclaim from Frost & Sullivan for its Dominance in the KSA Pharma Market with its Expanded Portfolio and Geographic Presence
Jamjoom Pharmaceuticals is dedicated to being a center of excellence in healthcare and providing high-quality, safe, and effective products to meet the regional need JEDDAH, Saudi Arabia, Aug. 19, 2020 /PRNewswire/ -- Based on its recent analysis of the KSA emerging generic pharmaceuticals marke...
athenahealth Earns the Frost & Sullivan 2020 RCM Company of the Year Award
athenahealth acclaimed by Frost & Sullivan for being at the forefront of solving critical RCM challenges and powering providers with agile solutions during the Covid-19 pandemic SANTA CLARA, California, Aug. 18, 2020 /PRNewswire/ -- Based on its recent analysis ofthe United States revenue cycle ...
Mindray Sets A New Standard for Infusion Systems with the BeneFusion n Series
SHENZHEN, China, Aug. 18, 2020 /PRNewswire/ -- Mindray (SZSE: 300760), a leading provider of medical devices and solutions, has released its new generation infusion system,BeneFusion n Series. By rethinking safety, simplicity, interoperability and data synergy, the BeneFusion n Series sets a new...
PharmAbcine announces the execution of a Material Cooperative Research and Development Agreement (MCRADA) with the NIH to evaluate the efficacy of PMC-403 to treat SCLS
DAEJEON, South Korea, Aug. 18, 2020 /PRNewswire/ -- PharmAbcine Inc. (KOSDAQ: 208340ks) entered into a Materials Cooperative Research and Development Agreement (MCRADA) with the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), in whic...
Global Cord Blood Corporation to Report First Quarter Fiscal 2021 Financial Results
HONG KONG, Aug. 18, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) (the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that it plans to release financial results fo...
Week's Top Stories
Most Reposted
Going Global: DCITS Embarks on International Expansion at Singapore Fintech Festival
[Picked up by 313 media titles]
2024-11-12 09:00DKSH Healthcare and Euris Unveil CRM & MCE Platform "ConnectPlus" to Revolutionize APAC Healthcare Distribution
[Picked up by 293 media titles]
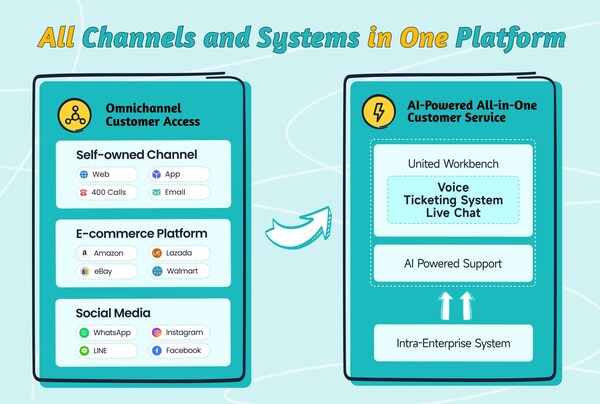
2024-11-13 09:00Sobot Introduces its All-in-One Solution at GITEX Global 2024
[Picked up by 291 media titles]
2024-11-12 11:00Ubiqconn Technology to Showcase Latest Marine Solutions at the 2024 International WorkBoat Show in New Orleans
[Picked up by 289 media titles]
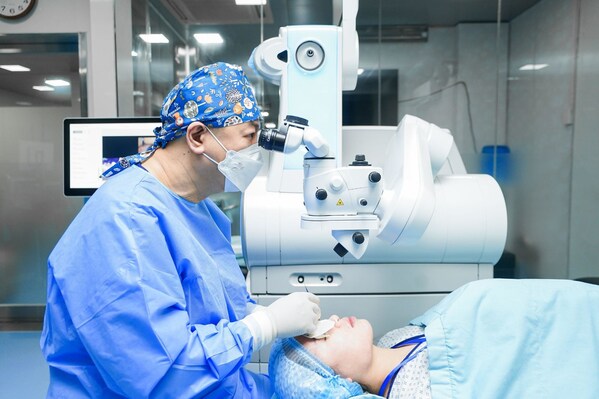
2024-11-11 21:00DND International Eye Hospital: Pioneering SMILE Pro Surgery & Leading the Trend of Refractive Surgery Tourism in Vietnam
[Picked up by 279 media titles]
2024-11-08 20:11