Medical/Pharmaceuticals
INOVIO's INO-5401 in Combination with PD-1 Inhibitor Libtayo(R) (cemiplimab) Demonstrates 85% of Newly Diagnosed Glioblastoma Patients Are Alive 12 Months Following Treatment
PLYMOUTH MEETING, Pennsylvania, May 14, 2020 /PRNewswire/ -- INOVIO (NASDAQ: INO) today announced that 85 percent (44 out of 52) of patients newly diagnosed with the deadly brain cancer glioblastoma multiforme (GBM) who received the company's DNA medicine INO-5401, in combination with INO-9012 an...
SciMount announces FDA approval of SMP-100's IND application for the treatment of Irritable Bowel Syndrome
CHENGDU, China, May 13, 2020 /PRNewswire/ -- SciMount Pharmatech, Co. Ltd., a wholly-owned subsidiary ofXiling Lab, announces today that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application for SMP-100 for the treatment of Irritable Bowel Synd...
Novartis Singapore returns Job Support Scheme payout worth SGD3.7 Million; aids community efforts against COVID-19
* Novartis Singapore commits to no retrenchments due to the COVID-19 pandemic, returnsSGD 3.7 million worth of Job Support Scheme (JSS) in April 2020 and declines any future payouts related to the pandemic. * The company has donated SGD 200,000 from the Novartis Global COVID-19 Response Fun...
Experts Underscore Essential Role for Patient Blood Management in and beyond the COVID-19 Pandemic
* Blood services and health authorities warn that a decline in donations due to COVID-19 could result in blood shortages, forcing frontline health care providers to face further difficult clinical decisions;[1],[2] * An international group of 43 medical experts points at a win-win concept to ...
Halodine(R) Nasal and Oral Antiseptics Show Rapid Antiviral Activity Against SARS-CoV-2 (COVID-19)
FORT LAUDERDALE, Florida, May 14, 2020 /PRNewswire/ -- Veloce BioPharma LLC reports today that their Halodine® Antiseptics have demonstrated rapid viricidal efficacy against SARS-CoV-2, the virus that causes COVID-19. Halodine® is a proprietary povidone-iodine antiseptic developed in partnership ...
TYVYT® (Sintilimab Injection) ORIENT-2 Study Met its Primary Endpoint of Overall Survival in the Second-Line Treatment of Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma
SUZHOU, China, May 14, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, autoimmune, metabolic and other major diseases, today jointl...
Hanwha Helps the Fight Against COVID-19 by Providing Life Park, a Smart Training Institute in the City of Yongin, Gyeonggi Province in Korea, as a Treatment Center
SEOUL, South Korea, May 14, 2020 /PRNewswire/ -- Hanwha is closely monitoring
the COVID-19 global health crisis and implementing various measures to protect
the health and safety of their employees and slow the spread of coronavirus by
helping hard-hit communities.
Breakthrough Innovation in Cancer Care From Merck Pipeline to Be Presented at ASCO 2020
- Results from two studies of BAVENCIO® to be featured in ASCO press briefing - Primary efficacy, biomarker and HRQoL analyses for tepotinib†, the first MET inhibitor to have received a regulatory approval for NSCLC withMET gene alterations - Two-year follow-up for first-in-class bifunctional im...
Hetero Enters Into a Licensing Agreement With Gilead Sciences, Inc. for the Manufacturing and Distribution of "Remdesivir" in 127 Countries, Including India, for COVID-19
HYDERABAD, India, May 14, 2020 /PRNewswire/ -- Hetero, one of India's leading generic pharmaceutical companies and the world's largest producer of anti-retroviral drugs, announced today that it has entered into a licensing agreement with Gilead Sciences, Inc. for the manufacturing and distributio...
Telehealth to Experience Massive Growth with COVID-19 Pandemic, Says Frost & Sullivan
Demand for telehealth will soar by 64.3% in the US in 2020 as the COVID-19 pandemic disrupts the practice of medicine and the delivery of healthcare SANTA CLARA, California, May 14, 2020 /PRNewswire/ -- Frost & Sullivan's recent analysis,Telehealth—A Technology-Based Weapon in the War Against the...
DERMALOG Fever Detection Protects Draegerwerk AG Production
- Despite Covid-19, assembly lines in many factories do not stop. Production at Draeger is also in full operation. As a manufacturer of ventilators, the German company's products are currently in high demand. To keep the risk of infection among employees as low as possible, Draeaeger has implemen...
First Patient Dosed with I-Mab's CD73 Antibody TJD5 in Phase 1/2 Clinical Trial in China for Advanced Solid Tumors
SHANGHAI and ROCKVILLE, Md., May 13, 2020 /PRNewswire/ -- I-Mab (NASDAQ: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel or highly differentiated biologics to treat diseases with significant unmet medical needs, today announ...
Fluxergy Announces $30 Million to Expand Manufacturing Capacity of Its One-hour Point-of-care Diagnostic Testing System in Response to COVID-19
IRVINE, California, May 13, 2020 /PRNewswire/ -- Irvine, California-based
Fluxergy LLC, a diagnostic test company, is making a$30 million investment to
expand its capability to scale production of the Fluxergy Analyzer diagnostic
testing system in response to the COVID-19 pandemic.
Ascletis' THR- beta Agonist ASC41 Receives Approval for Clinical Trials of NASH Indication
HANGZHOU, China and SHAOXING, China, May 13, 2020 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX code: 1672) announces today that it received IND approval from China's National Medical Products Administration (NMPA) for its in-house developed Category 1 Drug ASC41 to conduct clinical trials for Non-al...
CMAB Biopharma's development and manufacturing platform successfully completes Q-1802 bispecific antibody project for QureBio within 9 months
* CMAB completed a full scope development and manufacturing project for Q-1802 in 9 months for dual IND filing inChina and the US * Completion of the project demonstrates the significant speed to clinic achievable with CMAB's fully integrated development and manufacturing platform * QureBio'...
Cipla Enters Into a Licensing Agreement With Gilead to Expand Access to COVID-19 Treatment
MUMBAI, India, May 13, 2020 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) (and hereafter referred to as "Cipla") today announced that it has signed a non-exclusive licensing agreement with Gilead Sciences, Inc. for the manufacturing and distribution of the investigational medicine R...
Big Start helps businesses secure PPE gear as global demand spikes
PERTH, Australia, May 13, 2020 /PRNewswire/ -- Australian medical supply
innovator Big Start is being inundated with orders from around the world as
companies seek to protect staff as lockdowns are lifted and businesses get back
to work.
Big Start's
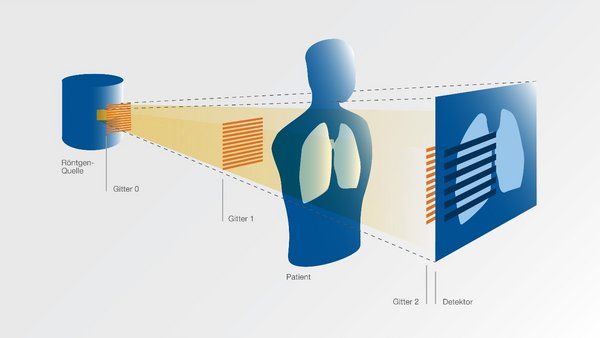
Low-dose radiographs could reveal typical lung changes
MUNICH, May 13, 2020 /PRNewswire/ -- Researchers at the Technical University of Munich (TUM) have developed an innovative x-ray method for lung diagnostics, which they now plan to test in one of its first applications for diagnosis of the respiratory ailmentCOVID-19 caused by Coronavirus. The met...
ChemPartner Assists the Mount Sinai Health System in Producing Reagent for COVID-19 Research
SHANGHAI, May 12, 2020 /PRNewswire/ -- Shanghai ChemPartner announced they have entered into a non-exclusive agreement with the Mount Sinai Health System ( Mount Sinai) to assist in a trial production run of a component of the Mount Sinai COVID-19 serological assay that recently received Emergency...
China Biologic Products to Report First Quarter 2020 Financial Results
BEIJING, China, May 12, 2020 /PRNewswire/ -- China Biologic Products Holdings, Inc. (NASDAQ: CBPO) ("China Biologic" or the "Company"), a leading fully integrated plasma-based biopharmaceutical company inChina, today announced that the Company plans to release its first quarter 2020 financial res...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 314 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00