Medical/Pharmaceuticals
Innovent Announces Financial Results for Full Year Ended December 31, 2019 and Corporate Progress
SUZHOU, China, March 30, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent" or "the Company") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality innovative medicines for the treatment of oncology, autoimmune, metabolic and oth...
INOVIO Interim Results of an Open-Label Phase 2 Trial of VGX-3100 Show Efficacy Against HPV-associated Vulvar Dysplasia
PLYMOUTH MEETING, Pennsylvania, March 30, 2020 /PRNewswire/ -- INOVIO Pharmaceuticals, Inc. (NASDAQ: INO) today reported interim results from an open-label Phase 2 trial designed to evaluate the safety and efficacy of VGX-3100 in women with vulvar dysplasia, also known as high grade squamous int...
OssDsign Receives Regulatory Approval and Prepares for Launch in Japan
STOCKHOLM, March 30, 2020 /PRNewswire/ -- OssDsign AB (publ) today announced that the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) has granted approval of OssDsign Cranial PSI for the Japanese market. OssDsign plans to launch OssDsign Cranial PSI inJapan together with a national dis...
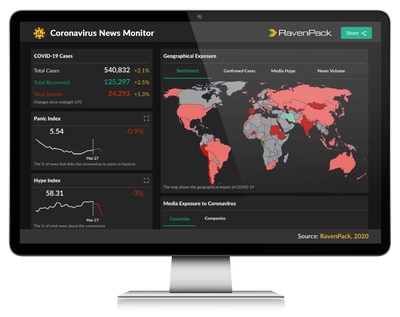
RavenPack Launches Free Coronavirus News Monitor to Help Data-driven Professionals Face the Challenging New Market Conditions
NEW YORK, March 30, 2020 /PRNewswire/ -- In response to the coronavirus pandemic, RavenPack, a leading provider of big data analytics, has launched a live and interactive website to help navigate the oceans of information currently available on the novel coronavirus and its impact on world affair...
Johnson & Johnson Announces a Lead Vaccine Candidate for COVID-19; Landmark New Partnership with U.S. Department of Health & Human Services; and Commitment to Supply One Billion Vaccines Worldwide for Emergency Pandemic Use
Johnson & Johnson and BARDA Together Commit More than $1 Billion to Novel Coronavirus Vaccine Research and Development; Company Expects to Initiate Phase 1 Human Clinical Studies of Vaccine Candidate at Latest bySeptember 2020 Johnson & Johnson Will Establish New U.S. Vaccine Manufacturing Capab...
Alphamab Oncology, Simcere and 3D Medicines Announce Partnership to Develop and Commercialize Subcutaneous Injectable anti-PD-L1 Antibody for Oncology Indications in Mainland China
SUZHOU, China, March 30, 2020 /PRNewswire/ -- Alphamab Oncology (stock code: 9966HK) announced today that Jiangsu Alphamab Biopharmaceuticals Co., Ltd. ("Jiangsu Alphamab"), a wholly-owned subsidiary of the Company, has established strategic partnership with Simcere and 3D Medicines (Beijing) Co....
SHL Medical acquires Weibel CDS
ZUG, Switzerland, March 30, 2020 /PRNewswire/ -- SHL Medical, a pioneer and technology frontrunner in the self-injection industry, announced its acquisition of Weibel CDS, a medical technology company offering products and solutions for drug delivery and novel packaging. Weibel was established i...
CSPC Pharmaceutical Announces 2019 Annual Results
Profit Attributable To Shareholders Increased 20.6% to RMB3,714 Million Innovative Drug Business Continued to Deliver Strong Growth New Products Launched Helped to Sustain Growth HONG KONG, March 30, 2020 /PRNewswire/ -- CSPC Pharmaceutical Group Limited (HKEx: 01093) ("CSPC Pharmaceutical" or the...
Vela Diagnostics to offer manual ViroKey™ SARS-CoV-2 RT-PCR Test after completion of Emergency Use Authorization validation, targeted to be complete by early April 2020
SINGAPORE, March 30, 2020 /PRNewswire/ -- Ahead of Emergency Use Authorization (EUA) and in accordance with the U.S. Food and Drug Administration (FDA) Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency guidance document issued on March 16, 2020, Vela Diag...
Nano Retina Announces Preliminary Results for First-in-Human Implantation of Its NR600 Artificial Retina Device
The device is designed to restore vision to persons blinded by age-related macular degeneration and retinitis pigmentosa First two patients to receive the device in March 2020 report visual effect HERZLIYA, Israel, March 30, 2020 /PRNewswire/ -- Nano Retina Ltd., developer of the NR600, an artif...
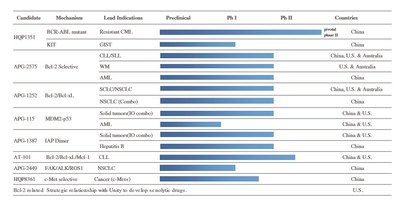
Ascentage Pharma Releases 2019 Annual Results, Reports Increased Investment in R&D and Advances in Global Clinical Development
SUZHOU, China and ROCKVILLE, MD, March 29, 2020 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today released its annual results for the...
GenScript ProBio Congratulates XlifeSciences on the Start of TAEST16001 Early Phase Clinical Trial
NANJING, China, March 27, 2020 /PRNewswire/ -- Guangzhou XlifeSciences, a partner of GenScript ProBio (GenScript's CDMO segment), announced that the early phase clinical trial of TAEST16001 project has been started. GenScript ProBio extends congratulations on this. This clinical trial project is...
GenScript ProBio Congratulates XlifeSciences on the Start of TAEST16001 Early Phase Clinical Trial
NANJING, China, March 27, 2020 /PRNewswire/ -- Guangzhou XlifeSciences, a partner of GenScript ProBio (GenScript's CDMO segment) announced that the early phase clinical trial of TAEST16001 project has been started. GenScript ProBio extends congratulations on this. This clinical trial project is ...
Foresee Pharmaceuticals and Accord Healthcare Announce MAA Submission for Camcevi(R) 42mg
TAIPEI, March 27, 2020 /PRNewswire/ -- Foresee Pharmaceuticals Co., Ltd. (6576.TWO) ("Foresee") and Accord Healthcare announced today that a Marketing Authorization Application (MAA) forCamcevi® 42mg (FP-001 LMIS 50mg), a ready-to-use 6-month depot formulation of leuprolide mesylate, has been su...
Alphamab Oncology Announces Clinical Supply Collaboration with Pfizer on KN026 in Combination with Ibrance(R) (palbociclib)
SUZHOU, China, March 27, 2020 /PRNewswire/ -- Alphamab Oncology (stock code: 9966HK) announced today that Jiangsu Alphamab Biopharmaceuticals Co., Ltd. ("Jiangsu Alphamab"), a wholly-owned subsidiary of the Company, has entered a clinical supply agreement with Pfizer Inc. ("Pfizer") to advance a ...
Luye Pharma Continues to Deliver Strong Growth in 2019
SHANGHAI, March 27, 2020 /PRNewswire/ -- Luye Pharma Group (02186.HK) reported its financial results for 2019 onMarch 27, 2020. According to the results, the company has achieved a total revenue ofRMB 6.358 billion, up 22.9% year-on-year. EBITDA wasRMB 2.488 billion, up 26.9% year-on-year; and n...
CStone submits new drug application for the targeted therapy avapritinib in Taiwan for the treatment of adults with advanced PDGFRA exon 18 mutant gastrointestinal stromal tumor
SUZHOU, China, March 27, 2020 /PRNewswire/ -- CStone Pharmaceuticals ("CStone" or the "Company", HKEX: 2616) today announced that the Company has submitted a New Drug Application (NDA) to the Taiwan Food and Drug Administration (TFDA) for avapritinib, a precision therapy being developed for the t...
C(2) PHARMA Opens Government and Research Access to Safety Stock of DIGOXIN API for Use in Potential COVID-19 Combination Treatment
LUXEMBOURG, March 27, 2020 /PRNewswire/ -- C2 PHARMA s.a.r.l. (C2P), a Luxembourg-based phytochemical and chemical pharmaceutical manufacturing and distribution group, is announcing that they will open access to safety stocks of the active pharmaceutical ingredient (API), Digoxin. In early March,...
Bosch Developing COVID-19-(SARS-CoV-2) Rapid Testing Kit for the Vivalytic Platform
WAIBLINGEN, Germany, March 27, 2020 /PRNewswire/ -- Bosch Healthcare Solutions is currently working on the development of a COVID-19 rapid testing kit for the Vivalytic platform and will launch this inApril 2020. The Vivalytic VRI test (Viral Respiratory Tract Infections) checks the patient's sam...
Fosun Pharma's Novel Coronavirus Nucleic Acid Detection Kit Receives Emergency Approval from China NMPA
SHANGHAI, March 26, 2020 /PRNewswire/ -- Shanghai Fosun Long March Medical Science Co., Ltd. ("Fosun Long March"), a wholly-owned subsidiary ofShanghai Fosun Pharmaceutical (Group) Co., Ltd ("Fosun Pharma"; Stock Code: 600196.SH, 02196.HK), has received emergency approval from the National Medica...
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00