Medical/Pharmaceuticals
Kazia Enters Clinical Collaboration With Cornell University for Phase II Clinical Study Using Paxalisib in Combination With Ketogenic Diet for Glioblastoma
SYDNEY, June 15, 2021 /PRNewswire/ -- Kazia Therapeutics Limited (NASDAQ: KZIA; ASX: KZA), an oncology-focused drug development company, is pleased to announce that it has entered a collaboration with theJoan & Sanford I Weill Medical College of Cornell University in the United States, to launch ...
Qynapse to present clinical results of QyScore® for Alzheimer's Disease and Multiple Sclerosis at the 7th Congress of the European Academy of Neurology
BOSTON, June 15, 2021 /PRNewswire/ -- QYNAPSE Inc., a medical technology company commercializing the most advanced artificial intelligence neuroimaging platform, today announced it will present clinical data on QyScore® for Alzheimer's disease and multiple sclerosis at the 7th Congress of the Eur...
Innovent Announces First Overweight or Obese Subject Dosed in A Phase 2 Clinical Trial of IBI362 (a GLP-1 and Glucagon Receptor Dual Agonist) in China
SAN FRANCISCO and SUZHOU, China, June 15, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major dise...
iAPPS Health Group, a Fin-MedTech company, announces former Senior Minister of State for Health Dr. Lam Pin Min as its Chairman
SINGAPORE, June 15, 2021 /PRNewswire/ -- iAPPS Health Group ("iHG") announced the appointment of former Senior Minister of State for Health, Dr. Lam Pin Min, as Chairman of its board of directors. Dr. Lam Pin Min brings with him a wealth of relevant experience as a veteran in the healthcare...
The U.S. FDA Approved IND Application to Investigate Combination of Asieris' APL-1202 and BeiGene's Tislelizumab as Neoadjuvant Therapy for MIBC Patients
SHANGHAI, June 15, 2021 /PRNewswire/ -- Asieris Pharmaceuticals (Asieris) today announced that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application of oral APL-1202 in combination with BeiGene's tislelizumab as neoadjuvant therapy in patients wit...
The U.S. FDA Approved IND Application to Investigate Combination of Asieris' APL-1202 and BeiGene's Tislelizumab as Neoadjuvant Therapy for MIBC Patients
SHANGHAI, June 14, 2021 /PRNewswire/ -- Asieris Pharmaceuticals (Asieris) today announced that the U.S. Food and Drug Administration (FDA) has approved the Investigational New Drug (IND) application of oral APL-1202 in combination with BeiGene's tislelizumab as neoadjuvant therapy in patients wit...
Ascentage Pharma Announces IND Clearance by the US FDA for Lisaftoclax (APG-2575) as Single Agent or in Combinations for the Treatment of ER+ Breast Cancer and Other Solid Tumors
SUZHOU, China, and ROCKVILLE, Md., June 15, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the Investigational Ne...
Novavax COVID-19 Vaccine Demonstrates 90% Overall Efficacy and 100% Protection Against Moderate and Severe Disease in PREVENT-19 Phase 3 Trial
* 93% efficacy against predominantly circulating Variants of Concern and Variants of Interest * 91% efficacy in high-risk populations * 100% efficacy against variants "not considered Variants of Concern/Interest" * All COVID-19 hospitalizations/death occurred in the placebo group * Company...
BioVaxys Receives Positive FDA Response For Pre-IND Review For CoviDTH Clinical Development
VANCOUVER, British Columbia, June 15, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA: 5LB) (OTCQB: BVAXF)("BioVaxys"), is pleased to announce today that the US Food and Drug Administration (FDA) has reviewed its Pre-IND request for a Type B review of its CoviDTH program and has d...
GENinCode Announces Major US Commercialisation Partnership With EVERSANA
OXFORD, England, June 15, 2021 /PRNewswire/ -- GENinCode UK Limited, the cardiovascular disease company focused on predictive genetics for the prevention of cardiovascular disease, announces its partnership with EVERSANA Life Sciences LLC ("EVERSANA") as its launch and commercialisation partner t...
Chinese biotech firm Vazyme to showcase full range of COVID-19 Testing Solutions at Medlab
DUBAI, UAE, June 14, 2021 /PRNewswire/ -- Chinese biotechnology company Vazyme will showcase a rich lineup of products at the 2021 Medlab Middle East, a leading medical laboratory exhibition co-located with Arab Health. The live in-person event is scheduled to take place from 21 to24 June, 2021 a...
Bridge Biotherapeutics Announces the Initiation of Patient Dosing in the Proof of Clinical Principle Study for BBT-401, an Investigational Drug for Ulcerative Colitis
SEONGNAM, South Korea, June 13, 2021 /PRNewswire/ -- Bridge Biotherapeutics (KQ288330) announced today the first patient dosing of BBT-401 at mid to high doses in its proof of clinical principle (PoCP) study to examine the drug's efficacy and safety in active ulcerative colitis (NCT04596293). Th...
ImmVira completed the first dosing for Phase II of MVR-T3011 (intratumoral injection) in the U.S. and China
SHENZHEN, China, June 11, 2021 /PRNewswire/ -- ImmVira announced that in the Phase II Clinical Trials of its leading oncolytic virus product, MVR-T3011* as intratumoral administration (MVR-T3011 IT), ImmVira has completed the first dosing in bothChina and the U.S. on May 28 2021 and June 11 20...
Tetracore, Inc. Introduces First USDA-Licensed Real-Time PCR Test for the Detection of Foot and Mouth Disease Virus in Bovine, Swine and Ovine
The Tetracore® Real-Time PCR Test was validated for use on epithelial and serum samples ROCKVILLE, Md., June 12, 2021 /PRNewswire/ -- Tetracore announced today the licensing of their VetAlert™ Foot and Mouth Disease Virus (FMDV) RNA Diagnostic Test Kit by the United States Department of Agricult...
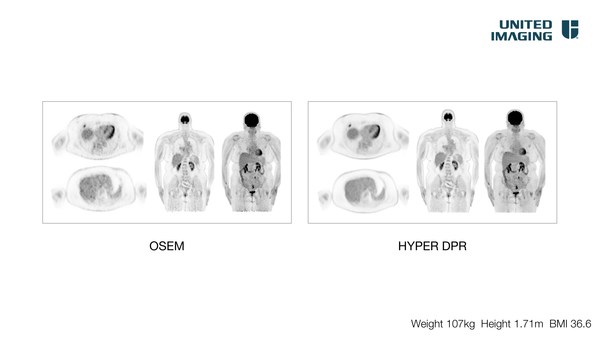
United Imaging Splashes Out at SNMMI 2021 With Clinical and Preclinical Technologies, Total-body AI, and Award-Winning Business Programs
HOUSTON, June 11, 2021 /PRNewswire/ -- United Imaging, a global leader in advanced medical imaging and radiotherapy equipment celebrating its tenth anniversary, announced a number of featured products and is highlighting several distinguished speakers at this year's SNMMI, where it is a Silver S...
Concord Medical Officially Opens New Specialty Cancer Hospital in Guangzhou
BEIJING, June 11, 2021 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specializing in cancer care, research and prevention by operating a network of medically advanced comprehensive cancer hospitals and standalone ...
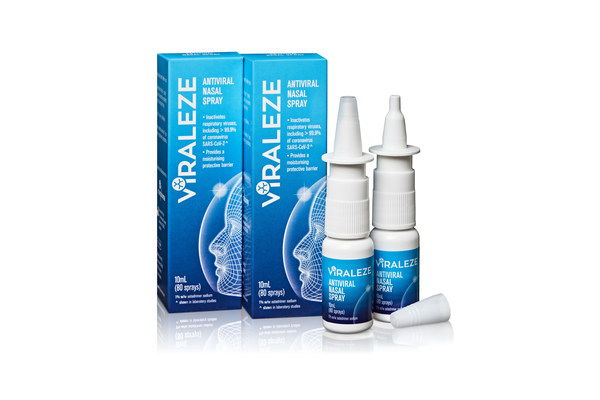
Viraleze registered for sale in Covid-ravaged India
MELBOURNE, Australia, June 11, 2021 /PRNewswire/ -- India's vast population
will have access from today to the Australian developed anti-COVID nasal spray
Viraleze™ following its registration in the country.
Viraleze registered for sale in Covid-ravaged India
MELBOURNE, Australia, June 11, 2021 /PRNewswire/ -- India's vast population
will have access from today to the Australian developed anti-COVID nasal spray
Viraleze™ following its registration in the country.
Long-lasting Chlorine Dioxide (ClO2) Aqueous Solution Presented by Taiko Pharmaceutical and Kitasato University Inactivates over 99.99% of SARS-CoV-2 (COVID-19 Virus)
OSAKA, Japan, June 11, 2021 /PRNewswire/ -- Taiko Pharmaceutical Co., Ltd. based inOsaka, Japan, hereby announced that long-lasting chlorine dioxide (ClO2) aqueous solution (*1) inactivated over 99.99% of SARS-CoV-2 (COVID-19 Virus), including its Alpha and Gamma variants (*2), in a test conducte...
Long-lasting Chlorine Dioxide (ClO2) Aqueous Solution Presented by Taiko Pharmaceutical and Kitasato University Inactivates over 99.99% of SARS-CoV-2 (COVID-19 Virus)
OSAKA, Japan, June 11, 2021 /PRNewswire/ -- Taiko Pharmaceutical Co., Ltd. based inOsaka, Japan, hereby announced that long-lasting chlorine dioxide (ClO2) aqueous solution (*1) inactivated over 99.99% of SARS-CoV-2 (COVID-19 Virus), including its Alpha and Gamma variants (*2), in a test conducte...
Week's Top Stories
Most Reposted
Edufrienz 99: One of Asia's First Digital Platform Advancing Character Education for Future-Ready, Compassionate Children
[Picked up by 314 media titles]
2026-01-23 11:03UOB prices AUD2 billion in five-year senior unsecured notes
[Picked up by 305 media titles]
2026-01-23 09:00AVPN's AI Opportunity Fund Expands Regional Efforts to Build AI Skilling Infrastructure for a Future-Ready Workforce Across Asia-Pacific
[Picked up by 302 media titles]
2026-01-21 12:39Government of Telangana and Blaize Sign MoU at Davos to Launch Telangana AI Innovation Hub and Advance Applied AI Initiatives
[Picked up by 297 media titles]
2026-01-22 15:16Klook's study of 374,000 reviews finds that lesser-known cities create deeper emotional connection with travelers
[Picked up by 280 media titles]
2026-01-20 15:40