Medical/Pharmaceuticals
MedAlliance Receives Fourth FDA Breakthrough Device Designation for Sirolimus Drug-Eluting Balloon in Treatment of De Novo Coronary Lesions
NYON, Switzerland, March 4, 2021 /PRNewswire/ -- MedAlliance, the first drug-eluting balloon (DEB) company in the world to receive US Food and Drug Administration (FDA) Breakthrough Device Designation Status for a sirolimus DEB, has now been awarded breakthrough status for SELUTION SLR™, its sust...
Medical expert warns thousands could die from HPV programming interrupted by Covid-19
LONDON, March 4, 2021 /PRNewswire/ -- On HPV Awareness Day, the International Papillomavirus Society is urging women to attend delayed cervical screenings and for all interrupted services to re-start as thousands continue to miss out on vital HPV care, with deadly consequences. The IPVS is also c...
Yuyu Pharma Publishes History for 80th Anniversary
SEOUL, South Korea, March 3, 2021 /PRNewswire/ -- Yuyu Pharma (KRX: 000220, CEO Robert Wonsang Yu), in anticipation of the 80th anniversary of its establishment onFebruary 28th, 2021, published its history of challenges and changes over the past 80 years. The history is told in storytelling forma...
WellmarkerBio receives approval for Phase I clinical trial from the Australian Therapeutic Goods Administration
SEOUL, South Korea, March 3, 2021 /PRNewswire/ -- Wellmarker Bio(www.wmbio.co
I-Mab to Report Financial Results for the Full Year 2020 and Provide Corporate Update on March 29, 2021
SHANGHAI and GAITHERSBURG, Md., March 3, 2021 /PRNewswire/ -- I-Mab (the "Company") (Nasdaq: IMAB), a clinical stage biopharmaceutical company committed to the discovery, development and commercialization of novel biologics, today announced that it will report financial results for the full year ...
Remote Pre-Approval Inspection Completed by EMA on WuXi Biologics' MFG4 Facility
WUXI, China, March 3, 2021 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, today announced that the European Medicines Agency (EMA) has completed a remote Pre-Approval Inspection (PAI) on its drug substance facility ...
SciClone Pharmaceuticals Officially Listed on the Main Board of the Hong Kong Stock Exchange
SHANGHAI, March 3, 2021 /PRNewswire/ -- SciClone Pharmaceuticals (Holdings) Limited ("SciClone Pharmaceuticals" or "SciClone") was officially listed today on the Main Board of The Stock Exchange of Hong Kong Limited ("Hong Kong Stock Exchange" or "HKEx") under stock code: 6600. In the initial of...
CMAB Biopharma Congratulates Partner Innovent Biologics on FDA Clearance of IND Application of its COVID-19 Antibody Project
SUZHOU, China, March 2, 2021 /PRNewswire/ -- CMAB Biopharma (Suzhou) Inc ("CMAB") congratulates its partner Innovent Biologics, Inc ("Innovent") (1801.HK) on the U.S. Food and Drug Administration (FDA) clearance of an Investigational New Drug (IND) application for its antibody candidate which ta...
Seegene's Latest COVID-19 Test Can Simultaneously Target 4 Genes of SARS-CoV-2 and Recognize Multiple Virus Variants
* Seegene's new product filters COVID-19 and variants during primary PCR testing in 1 hr 55 minutes * Seegene's proprietary high multiplex technology enables detection of 10 different targets * S. Korean firm to research additional versions of variant diagnostic tests SEOUL, South Korea,...
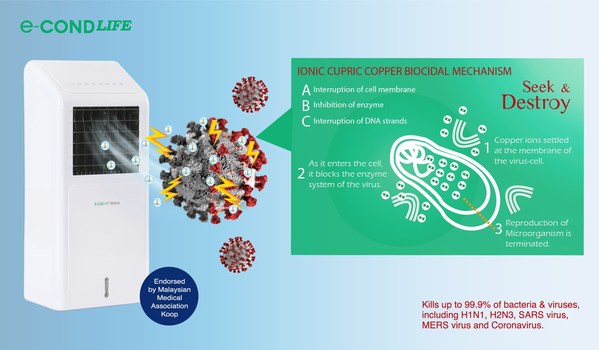
e-condLIFE: Cost of human life and business shut down can be minimized with this new prevention technology
* A smarter preventative way to keep family members safe * Peace of mind for staff and customers that the premises are being disinfected 24 hours KUALA LUMPUR, Malaysia, March 3, 2021 /PRNewswire/ -- Since launching inJune 2020, e-condLIFE has been well received and proven to be effective. Dr...
AGC Biologics Announces the Expansion of Their Cell and Gene Therapy Facility in Milan, Italy
SEATTLE, March 3, 2021 /PRNewswire/ -- AGC Biologics, a leading global Biopharmaceutical Contract Development and Manufacturing Organization (CDMO), announced plans for the expansion of their Cell and Gene Therapy Center of Excellence inMilan, to increase capacities and implement viral vector su...
VUNO presents its state-of-the-art AI medical imaging technology at ECR 2021
SEOUL, South Korea, March 2, 2021 /PRNewswire/ -- VUNO Inc., South Korean AI medical imaging company, will showcase its complete deep learning-based imaging offerings two years in a row in its virtual booth at the 2021 European Congress of Radiology (ECR), one of the biggest annual events, featur...
Cipla Gulf Expands Partnership with Alvotech for Commercialization of Biosimilars in Australia and New Zealand
MUMBAI, India, March 2, 2021 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) referred to as "Cipla" today announced that its subsidiary, Cipla Gulf FZ LCC ("Cipla Gulf') is expanding its partnership with Alvotech for the marketing and distribution of four biosimilar medicines inAustra...
Ambu Acclaimed by Frost & Sullivan for Its Groundbreaking Single-use Flexible Endoscopes
Ambu's customer-focused innovations and use of advanced technologies help the company address market needs in a wide range of applications SANTA CLARA, Calif., March 2, 2021 /PRNewswire/ -- Based on its recent analysis of the global flexible single-use endoscope market for advanced visualization...
Cloud-based Enterprise Imaging to Help Expedite Healthcare Providers' Journey of Enabling Better Care
SANTA CLARA, Calif., March 2, 2021 /PRNewswire/ -- Responding to the shift toward value-based care, provider M&A and consolidation, data expansion, and analytics innovations, healthcare providers are accelerating their digital transformation for greater efficiency, automation and access across cl...
Global Cord Blood Corporation Announces Receipt of Unsolicited Non-Binding Acquisition Proposal
HONG KONG, March 2, 2021 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) ("GCBC" or the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that its board of directors (the "...
Merck and GPHL Collaborate on Business Innovation and Development in the Greater Bay Area
GUANGZHOU, China, March 2, 2021 /PRNewswire/ -- Merck, a leading science and technology company, today signed a Memorandum of Understanding (MOU) to begin strategic collaboration with Guangzhou Pharmaceutical Holdings Limited (GPHL), China's leading pharmaceutical company. The collaboration aim...
Cipla receives final approval for generic version of GlaxoSmithKline's IMITREX® (Sumatriptan Nasal Spray, 20 mg)
MUMBAI, India, March 2, 2021 /PRNewswire/ -- Cipla Limited (BSE: 500087) (NSE: CIPLA EQ) (hereinafter referred to as "Cipla") today announced that it has received final approval for its Abbreviated New Drug Application (ANDA) for Sumatriptan Nasal Spray, 20 mg from the United States Food and Drug...
Oscotec and Beactica Therapeutics announce license and collaboration agreement to develop new cancer drug
STOCKHOLM, March 2, 2021 /PRNewswire/ -- Oscotec Inc. (039200: KOSDAQ), the Korean drug development company, and Beactica Therapeutics AB, the Swedish drug discovery company, today announced a new research development and licensing agreement. Oscotec and Beactica will initially jointly collaborat...
CMAB Biopharma Congratulates QureBio on FDA Clearance of IND Application for Claudin18.2/PD-L1 Bispecific Antibody
SUZHOU, China, March 2, 2021 /PRNewswire/ -- Recently, CMAB Biopharma (Suzhou) Inc's ("CMAB") partner QureBio Ltd ("QureBio") has announced its innovative drug Q-1802 received United States Food and Drug Administration (FDA) clearance for an Investigational New Drug (IND) application. This applic...
Week's Top Stories
Most Reposted
Visa Supports Chinese Cardholders to Add Cards to Apple Pay for a More Convenient and Secure Payment Experience
[Picked up by 310 media titles]
2026-01-15 13:00JIOS Aerogel® Secures Hyundai Contract Through New Licensing Model
[Picked up by 296 media titles]
2026-01-15 10:00Infobip strengthens leadership to drive growth and innovation
[Picked up by 281 media titles]
2026-01-16 11:00Klook's study of 374,000 reviews finds that lesser-known cities create deeper emotional connection with travelers
[Picked up by 279 media titles]
2026-01-20 15:40Docquity Launches Engage™, an AI and Insights-Powered HCP Intelligence Engine for the Life Sciences Industry
[Picked up by 278 media titles]
2026-01-15 13:00