Medical/Pharmaceuticals
Seoul National University Bundang Hospital Establishes the first "International PSP Research and Treatment Center" in Korea
* MOU Signed Among SNUBH-CurePSP-GemVax for the Establishment of International PSP Research and Treatment Center * Tackling Global Challenges in an Aging Era… "Aspiring to become a global research and treatment hub for the rare neurodegenerative disease" SEOUL, South Korea, Dec. 9, 2024 /PRNe...
Lunit Marks Go-Live in Groundbreaking National AI Cancer Screening Program in BreastScreen NSW
Lunit INSIGHT MMG integrated into the clinical workflow of Australia's BreastScreen New South Wales to assist in reading approximately 31,000 mammography exams annually SEOUL, South Korea, Dec. 9, 2024 /PRNewswire/ -- Lunit (KRX:328130.KQ), a leading provider of AI-powered solutions for cancer d...
WAT Medical Sponsors American Headache Society 2024 Scottsdale Headache Symposium
VANCOUVER, BC, Dec. 9, 2024 /PRNewswire/ -- WAT Medical Enterprise proudly sponsored the 2024 Scottsdale Headache Symposium, organized by the American Headache Society fromNovember 14-17 in Scottsdale, AZ. This annual event brought together a multidisciplinary audience of neurologists, general p...
Actinogen randomizes first US participant in XanaMIA phase 2b/3 Alzheimer's disease trial
* Late-stage trial in Alzheimer's opens 10 sites in US, with results due in 2025 and 2026 * Appoints US-based Chief Commercial Officer, Andrew Udell * Xanamem's unique ability to control brain cortisol draws widespread coverage in the Australian media. SYDNEY, Dec. 9, 2024 /PRNewswire/ -- A...
Concord Healthcare Announces Approval Granted to the Application for the Proton Therapy Equipment Registration Certificate
BEIJING, Dec. 9, 2024 /PRNewswire/ -- Concord Medical Services Holdings Limited ("Concord Medical" or the "Company") (NYSE: CCM), a healthcare provider specialized in cancer treatment, research, education and prevention inChina, today announced that the National Medical Products Administration of...
Dizal Presents Latest Data of DZD8586, a LYN/BTK Dual Inhibitor, in B-cell Non-Hodgkin Lymphoma at the 2024 ASH Annual Meeting
DZD8586 demonstrated encouraging anti-tumor activity with manageable safety and favorable pharmacokinetic (PK) characteristics in patients with B-NHL SHANGHAI, Dec. 9, 2024 /PRNewswire/ -- Dizal (SSE:688192), a biopharmaceutical company committed to developing novel medicines for the treatment o...
Dr. James G. Fujimoto of U.S. Awarded 45th Honda Prize for His Team's Development of Optical Coherence Tomography (OCT): Real-time Tissue Imaging Technology
- OCT Marks Breakthrough in Disease Detection and Manufacturing - TOKYO, Dec. 9, 2024 /PRNewswire/ -- The Honda Foundation in Tokyo held the 45th Honda Prize Award Ceremony onNovember 18, 2024, where this year's prize was awarded to Dr.James G. Fujimoto, Elihu Thomson Professor of Electrical Eng...
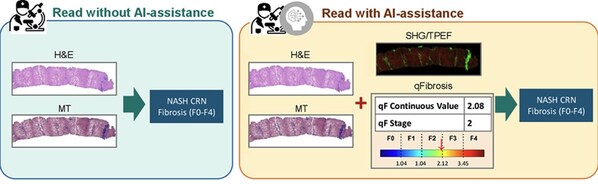
BRIDGING CLINICAL RESEARCH AND CLINICAL CARE WITH AI-AIDING PATHOLOGISTS TOOL
SINGAPORE, Dec. 9, 2024 /PRNewswire/ -- A newly published collaborative study
2024 ASH Oral Presentation----Abbisko presents promising preliminary phase 2 study results of pimicotinib in the treatment of Chronic Graft-versus-Host Disease (cGvHD) at the 66th ASH Annual Meeting
SHANGHAI, Dec. 8, 2024 /PRNewswire/ -- December 8, 2024, Abbisko Therapeutics (HKEX: 02256) announced the presentation of preliminary Phase 2 study results for pimicotinib (ABSK021) in patients with chronic Graft-versus-Host Disease (cGvHD) who have either progressed or not responded to one or mo...
Jacobio Pharma Presented preliminary Data on BET Inhibitor for Myelofibrosis at 2024 ASH
BEIJING, SHANGHAI and BOSTON, Dec. 8, 2024 /PRNewswire/ -- Jacobio Pharma (1167.HK), a clinical-stage oncology company dedicated to developing therapies toward undruggable targets, today presented preliminary Phase I data of BET inhibitor JAB-8263 to treat myelofibrosis (MF) at the 2024 ASH (Ame...
Encouraging Efficacy and Safety: CStone Presents Latest Clinical Data on CS5001 for Advanced Lymphoma at the 66th ASH Annual Meeting
* CS5001 is so far the first anti-ROR1 ADC known to show clinical anti-tumor activity in both solid tumors and lymphomas. The data presented at ASH highlighted the latest safety and efficacy of CS5001 as a monotherapy for patients with advanced lymphomas. * CS5001 is well tolerated in heavily...
Innovent Presents Updated Data From Innovative Anti-CLDN18.2 ADC (IBI343) Phase 1 Study in Patients with Advanced Pancreatic Cancer at the ESMO Asia Congress 2024
SAN FRANCISCO and SUZHOU, China, Dec. 9, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, cardiovascular and metabolic, autoimmune,...
EpiVax Drives Immunogenicity Innovation in 2024: Year in Review
PROVIDENCE, R.I., Dec. 7, 2024 /PRNewswire/ -- EpiVax, Inc., a leader in preclinical immunogenicity risk assessment for biologic and peptide therapeutics, celebrates a productive 2024 marked by scientific innovation, service diversification, and corporate growth. This year, EpiVax expanded the b...
Caliway Biopharmaceuticals Included in FTSE TWSE Taiwan Eight Industries and Mid-Cap 100 Indices
NEW TAIPEI CITY, Dec. 6, 2024 /PRNewswire/ -- Caliway Biopharmaceuticals Co., Ltd. (TWSE-6919) is proud to announce its inclusion in the FTSE TWSE Taiwan Mid-Cap 100 Index and the FTSE TWSE Taiwan Eight Industries Index, two prestigious benchmarks jointly compiled by FTSE Russell and the Taiwan S...
Another star technology platform of the Sanyou AI-STAL family
——Introducing Sanyou Mrna Mab innovative antibody generation technology platform SHANGHAI, Dec. 6, 2024 /PRNewswire/ -- With the announcement of the 2024 Nobel Prize in Physiology or Medicine, microRNA has once again become the focus of global attention. In the past 20 years, three Nobel Prizes h...
Singapore's IMAGINE AI: Largest Global Gathering to Shape the Future of Healthcare with AI Innovations
SINGAPORE, Dec. 6, 2024 /PRNewswire/ -- IMAGINE AI is the largest calendar event inSingapore that brings together the brightest minds in healthcare and artificial intelligence to explore the transformative potential of AI in healthcare. Hosted at the Marina Bay Sands Expo & Convention Centre, thi...
SD Biosensor Signs Tripartite MOU for R&D of New Products for Extensively Drug-Resistant Tuberculosis with Japan's RIT/JATA and Korea's International Tuberculosis Research Center
* SD Biosensor strengthens its new product lineup with the POC molecular diagnostic platform M10 * Early detection of patients is expected to contribute to the eradication of tuberculosis in high-risk Asian countries SEOUL, South Korea, Dec. 6, 2024 /PRNewswire/ -- SD Biosensor, Inc. (KQ13731...
Alamar Biosciences Establishes Commercial Presence in APAC Region with First ARGO HT Install at Hong Kong Center for Neurodegenerative Diseases
FREMONT, Calif., Dec. 6, 2024 /PRNewswire/ -- Alamar Biosciences, a company powering precision proteomics to enable the earliest detection of disease, is proud to announce its commercial presence in theAsia Pacific (APAC) region with the installation of the ARGO™ HT System, an advanced ultra-sens...
Ono Enters into Drug Discovery Collaboration Agreement with Congruence Therapeutics to Generate Novel Small Molecule Correctors in the Oncology Area
OSAKA, Japan and MONTREAL , Dec. 5, 2024 /PRNewswire/ -- Ono Pharmaceutical Co., Ltd. (Headquarters:Osaka, Japan; President: Toichi Takino; "Ono") announced that it has entered into a drug discovery collaboration agreement with Congruence Therapeutics (Headquarters:Montreal, Quebec, Canada; CEO:...
KANEKA UBIQUINOL™ AWARDED PRESTIGIOUS COMPLEMENTARY MEDICINES AUSTRALIA RAW MATERIAL SUPPLIER OF THE YEAR AWARD 2024
SYDNEY, Dec. 6, 2024 /PRNewswire/ -- Kaneka Ubiquinol™ has been awarded the
covetedRaw Material Supplier of the Year Award 2024 at the Complementary
Medicines Australia (CMA) Awards, held inSydney this evening.
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 309 media titles]
2026-02-03 11:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 302 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 286 media titles]
2026-02-01 19:35