Medical/Pharmaceuticals
Telix Q3 2024 Business Update - Quarterly Revenue Exceeds AU$200M
MELBOURNE, Australia, Oct. 17, 2024 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, Telix, the Company) today provides an update on its revenue and operational performance for the quarter ended30 September 2024 (Q3 2024). Revenue update, full year guidance reaffirmed * Unaudited to...
KIND Announces Late-Breaking Abstract Accepted for Presentation on AND017 Clinical Trials to Treat Anemia in Chronic Kidney Disease (CKD) at ASN - Kidney Week 2024
SAN FRANCISCO, Oct. 17, 2024 /PRNewswire/ -- Kind Pharmaceutical ("Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC"), a clinical-stage biopharmaceutical company focused on developing innovative medicines to treat hematological diseases and cancers, today announced that the late-br...
Cambrex Opens New Stability Storage Facility in Durham, North Carolina
EAST RUTHERFORD, N.J., Oct. 17, 2024 /PRNewswire/ -- Cambrex, a leading global contract development and manufacturing organization (CDMO), today announced that their stability storage business, Q1 Scientific, has opened a new cGMP facility inDurham, North Carolina, expanding its capacity for envi...
CSL Vifor and Travere Therapeutics Announce Swissmedic approval of FILSPARI® (sparsentan) for the treatment of IgA Nephropathy
Temporary marketing authorization is based on statistically significant and clinically meaningful results from the phase-III PROTECT trial ST. GALLEN, Switzerland and SAN DIEGO, Oct. 17, 2024 /PRNewswire/ -- CSL Vifor and Travere Therapeutics, Inc., (NASDAQ: TVTX) today announced that Swissmedic...
IND Application for a Phase Ⅲ Clinical Study of KN026 Combined with Albumin-bound Docetaxel as Neoadjuvant Treatment for Breast Cancer was Approved
SUZHOU, China, Oct. 17, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966 HK) and CSPC Pharmaceutical Group Co., Ltd. ("CSPC") (stock code: 1093.HK) jointly announced that an application for a phase III clinical study (Study ID: KN026-004) of the anti-HER2 bispecific antibody KN026 combine...
Innovent Announces Phase 2 Clinical Study of Picankibart (IBI112) in Chinese Patients with Ulcerative Colitis Met Primary Endpoint
SAN FRANCISCO and SUZHOU, China, Oct. 17, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabo...
Presagen's AI fertility product Life Whisperer acquired by Astec
ADELAIDE, Australia, Oct. 17, 2024 /PRNewswire/ -- Astec, a global manufacturer of medical equipment for assisted reproduction, has acquired Presagen's fertility product Life Whisperer, a non-invasive and rapid embryo and egg (oocyte) assessment tool designed to improve pregnancy outcomes for IVF...
Mars announces new leadership for its Veterinary Health and Science & Diagnostics businesses
* Nefertiti Greene announced as Global President of Mars Veterinary Health * Katie Devine joining to lead Mars Science & Diagnostics * Devine brings over 25 years' experience in consumer health and pharma * Mars has been a global leader in pet care for almost 90 years, now spanning veterina...
EmeTerm Smart and HeadaTerm 2 Achieve Health Canada MDL Certification
VANCOUVER, BC, Oct. 16, 2024 /PRNewswire/ -- WAT Medical Enterprise proudly announces a significant achievement: both EmeTerm Smart and HeadaTerm 2 have received Medical Device Licences (MDL) from Health Canada. EmeTerm Smart was certified onAugust 12, 2024, followed by HeadaTerm 2 on September 2...
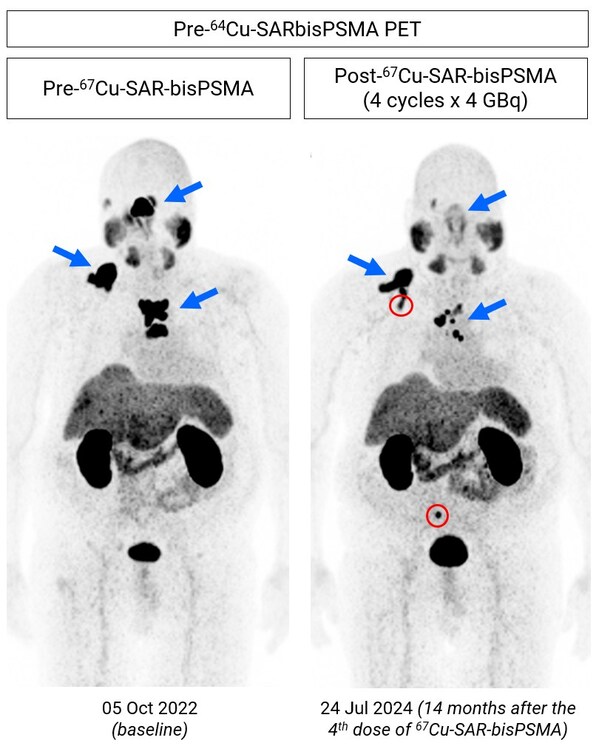
Copper-67 SAR-bisPSMA updates
SYDNEY, Oct. 16, 2024 /PRNewswire/ -- HIGHLIGHTS Cohort 4 - SECuRE Trial * The third participant of cohort 4 (multi-dose) of the SECuRE trial1 has now completed the Dose Limiting Toxicity (DLT) period after a second dose of 12GBq of67Cu-SAR-bisPSMA, following on from the announcement dated 1...
Tanner Health and Healthliant Ventures Partner With Corti to Accelerate Medical Coding, Cutting Workload by 80 Percent
CARROLLTON, Ga., Oct. 16, 2024 /PRNewswire/ -- In a move set to transform healthcare operations acrossGeorgia and Alabama, Tanner Health and Healthliant Ventures have announced a strategic partnership with Corti, the trusted AI platform to healthcare systems worldwide. The collaboration is expect...
IGCS Late Breaking Abstract and The Lancet: Akeso Published Positive PFS and OS Results from Phase 3 First-Line Study of Cadonilimab in Cervical Cancer
HONG KONG, Oct. 16, 2024 /PRNewswire/ -- Akeso Biopharma (9926.HK) (" Akeso", the "Company" ) announced positive results in progression-free survival (PFS) and overall survival (OS) from its Phase 3 clinical study (COMPASSION-16/AK104-303). This study evaluated the efficacy of its independently...
TenNor Announces More than 300 Million RMB Financing to Support Development and Commercialization of Late-Stage Assets Including Rifasutenizol for Heliobacter pylori Infections
SUZHOU, China, Oct. 16, 2024 /PRNewswire/ -- TenNor Therapeutics, a clinical stage company dedicated to developing new therapies to address unmet needs in infectious diseases, announced today the initial closing of a Series E financing round for more than300 million RMB. New investor AMR Action F...
Join the GenScript Biotech Global Forum: Unlocking Innovations in Cell and Gene Therapies
PISCATAWAY, N.J., Oct. 16, 2024 /PRNewswire/ -- The global cell and gene therapy (CGT) industry is poised for remarkable growth, driven by significant advances in life sciences and robust investments from capital markets. To strengthen this vital sector, GenScript is bringing together leading sc...
SONIRE's HIFU Therapy System Designated as Breakthrough Device by FDA
TOKYO, Oct. 16, 2024 /PRNewswire/ -- SONIRE Therapeutics Inc. (hereinafter referred to as "SONIRE"), based inTokyo, Japan, announces that its self-developed next-generation HIFU (High-Intensity Focused Ultrasound) therapy system (development code: Suizenji) has been designated as a breakthrough ...
Frost & Sullivan grants the 2024 Global Pluripotent stem cell drug R&D Innovation Award to ZEPHYRM BIOTECHNOLOGIES
SHANGHAI, Oct. 16, 2024 /PRNewswire/ -- The 18th The Growth Innovation Leadership (GIL) Council & the 3rd New Investment Conference in 2024 was held in Shanghai from August 28 to 30, 2024. On the evening of August 28, the prestigious 2024 Global and China Awards for Growth, Innovation, and Leaders...
Members Health Calls for National Reform to Strengthen Healthcare and Bolster Economic Prosperity and Social Wellbeing
MELBOURNE, Australia, Oct. 16, 2024 /PRNewswire/ -- Members Health is calling for major reforms to boost health care accessibility and affordability and improve transparency of hospitals and doctors acrossAustralia as part of a landmark report released today for the not-for-profit health insuranc...
Cardiex Awarded $525,000 Grand Prize in NIH RADx Tech for Maternal Health Challenge
SYDNEY, Oct. 16, 2024 /PRNewswire/ -- Cardiex, a global health technology company specializing in cardiovascular diagnostics and devices, today announced it has been awarded the$525,000 grand prize in the US National Institutes of Health (NIH) RADx Tech for Maternal Health Challenge. The award re...
AtomVie Global Radiopharma and Radiopharm Theranostics Partner to Develop and Manufacture 177Lu-BetaBart Radioantibody for Treatment of Multiple Solid Tumors
HAMILTON, ON, Oct. 16, 2024 /PRNewswire/ -- AtomVie Global Radiopharma (AtomVie), a leading radiopharmaceutical Contract Development and Manufacturing Organization (CDMO), has entered into an agreement with Radiopharm Ventures (RV), a Joint Venture between Radiopharm Theranostics (RAD) and MD And...
PharmAust affirms corporate strategy with name change to Neurizon Therapeutics
MELBOURNE, Australia, Oct. 15, 2024 /PRNewswire/ -- Neurizon Therapeutics Limited (ASX: NUZ) ("Neurizon" or "the Company"), a clinical-stage biotech company dedicated to advancing treatments for neurodegenerative diseases, is pleased to announce it has officially changed its name from PharmAust L...
Week's Top Stories
Most Reposted
Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 323 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 315 media titles]
2026-02-03 11:00Mastercard Launches Portfolio of Fleet Solutions in Asia Pacific
[Picked up by 311 media titles]
2026-02-04 09:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 305 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 288 media titles]
2026-02-01 19:35