Medical/Pharmaceuticals
Kazia Therapeutics Regains Full Nasdaq Listing Compliance
Restoration of Nasdaq compliance follows $50 million institutional financing and reinforces balance-sheet strength SYDNEY, Dec. 22, 2025 /PRNewswire/ -- Kazia Therapeutics Limited ("Kazia" or the "Company") (NASDAQ: KZIA), a clinical-stage oncology company focused on developing innovative therap...
Guardant Health Japan receives regulatory approval of Guardant360® CDx liquid biopsy as companion diagnostic for imlunestrant in metastatic or recurrent breast cancer previously treated with endocrine therapy
Guardant360® CDx is the first companion diagnostic to be approved in Japan to identify ESR1 mutations in patients with hormone receptor-positive, HER2-negative breast cancer for treatment with imlunestrant TOKYO, Dec. 22, 2025 /PRNewswire/ -- Guardant Health Japan Corp. today announced that the...
Menarini Silicon Biosystems announces PACE trial biomarker analysis results confirming clinical utility of CELLSEARCH® CTC enumeration to guide treatment decisions in a specific metastatic breast cancer subtype
The secondary analysis of the PACE trial highlights the value of counting Circulating Tumor Cells (CTCs) in the blood to help guide treatment escalation or de-escalation in patients with hormone receptor-positive (HR+), HER2-negative (HER2-) metastatic breast cancer whose disease progressed after...
The 8th Hainan International Health Industry Expo opens in Sanya
HAIKOU, China, Dec. 22, 2025 /PRNewswire/ -- A report from Hainan International Media Center: On December 20, 2025, the 8th Hainan International Health Industry Expo opened in Sanya. Coinciding with the crucial juncture of the Hainan Free Trade Port's island-wide special customs operations, this...
Fosun International Honored with TVB's Highest Accolade, "Outstanding ESG Award"
HONG KONG, Dec. 22, 2025 /PRNewswire/ -- On 19 December 2025, the TVB ESG Awards 2025 Presentation Ceremony, hosted by Television Broadcasts Limited ("TVB"), was held at the Hong Kong Convention and Exhibition Center. Fosun International was honored with the highest accolade, the "Outstanding ESG...
Wong To Yick Unveils Revitalised Official Website
Launching a New No.1 Brand Chapter with Ambassador Joey Yung
Enhanced Authenticity Guidance & Usage Education for Elevated Consumer
Confidence
[High-resolution promotional photo download: Here
Precision Medicine Portfolio Update: Illuccix China Phase 3 Study, TLX101-CDx and TLX250-CDx FDA Resubmissions
MELBOURNE, Australia and INDIANAPOLIS, Dec. 22, 2025 /PRNewswire/ -- Telix Pharmaceuticals Limited (ASX: TLX, NASDAQ: TLX, "Telix") today provides a precision medicine portfolio update in relation to: * TLX591-CDx (Illuccix® in approved jurisdictions, 68Ga-PSMA-11): Positive data from Phase ...
T-MAXIMUM Pharmaceutical's Allogeneic CAR-T Therapy MT027 Receives FDA IND Clearance to Proceed to Phase II clinical Trial for Recurrent Glioblastoma
BEIJING, Dec. 21, 2025 /PRNewswire/ -- T-MAXIMUM Pharmaceutical announced that its proprietary allogeneic, B7-H3-targeted CAR-T therapy, MT027, has received IND Clearance from the U.S. Food and Drug Administration (FDA) to initiate a Phase II clinical trial for the treatment of recurrent glioblas...
Jacobio Pharma Enters Global Exclusive License Agreement with AstraZeneca for Pan-KRAS Inhibitor JAB-23E73
BEIJING, SHANGHAI and BOSTON, Dec. 21, 2025 /PRNewswire/ -- Jacobio Pharma (1167.HK,) today announced that it has entered an agreement with AstraZeneca for its proprietary Pan-KRAS inhibitor JAB-23E73. AstraZeneca will receive exclusive development and commercialisation rights outside ofChina, wh...
Alphamab Oncology Announces Biparatopic HER2-targeting ADC JSKN003 Was Granted Breakthrough Therapy Designation by the FDA for the Treatment of PROC
SUZHOU, China, Dec. 20, 2025 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the biparatopic HER2-targeting antibody-drug conjugate (ADC) JSKN003, independently developed by the Company, and co-developed with JMT-Bio Technology Co., Ltd., a wholly-owned subsidiary of CSPC P...
Gene Solutions Concludes Successful ESMO Asia Congress 2025 with AI-Powered Multi-Omics Liquid Biopsy Symposium and Two Best Poster Awards
SINGAPORE, Dec. 20, 2025 /PRNewswire/ -- Gene Solutions, a pioneering genetic testing company, announced key achievements at the European Society for Medical Oncology (ESMO) Asia Congress 2025—held inSingapore on December 5–7. Highlights included a full-house symposium, seven scientific abstracts...
Woori IO Shareholders Approve Share Exchange to Become Wholly-Owned Subsidiary of OSR Holdings
Company advancing dual clinical pathways, including a Samsung-supported PoC trial in Korea and a planned U.S. FDA trial with a leadingCalifornia research university BELLEVUE, Wash., Dec. 19, 2025 /PRNewswire/ -- OSR Holdings, Inc. (NASDAQ: OSRH) ("OSR Holdings"), a global healthcare holding comp...
Pictor's Test Poised to Transform Hepatitis B Immunity Testing Amid CDC Guidance Shifts
CARLSBAD, Calif., Dec. 19, 2025 /PRNewswire/ -- With the CDC's Advisory
Committee on Immunization Practices (ACIP) recommending post-vaccination
antibody testing for hepatitis B, laboratories are facing new demands to assess
immunity more effectively. Pictor
WuXi Biologics Achieves CDP Highest "A" Ratings in Both Climate Change and Water Security
SHANGHAI, Dec. 19, 2025 /PRNewswire/ -- WuXi Biologics (2269.HK) announced that it has been named to the CDP "A" lists for both Climate Change and Water Security for 2025, underscoring its leadership in environmental stewardship and transparent disclosure. Climate Change Leadership Being recogn...
NEW DRUG APPLICATION FOR TINENGOTINIB TABLETS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION
NANJING, China and GAITHERSBURG, Md., Dec. 18, 2025 /PRNewswire/ -- TransThera Sciences Nanjing, Inc. (the "TransThera") announced that the new drug application for Tinengotinib tablets has been accepted by the Center for Drug Evaluation ("CDE")of the National Medical Products Administration ("NM...
MEDIPOST Announces the Exclusive Commercialization License Agreement with Teikoku Seiyaku for Knee Osteoarthritis Treatment CARTISTEM® in Japan
- South Korean biotech company secures US$8 million in upfront payment for the joint commercialization of the stem cell therapy CARTISTEM® targeting the Japanese knee osteoarthritis market. SEOUL, South Korea, Dec. 19, 2025 /PRNewswire/ -- MEDIPOST Co., Ltd. (KOSDAQ 078160), a fully integrated c...
Neuraxpharm Goes Live with TraceLink MINT to Standardize Real‑Time Data Exchange Across Its Global Supply Chain Network
The European specialty pharmaceutical company establishes a unified digital data foundation to enable future agentic orchestration BOSTON, Dec. 18, 2025 /PRNewswire/ -- TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the supply chain, today announced t...
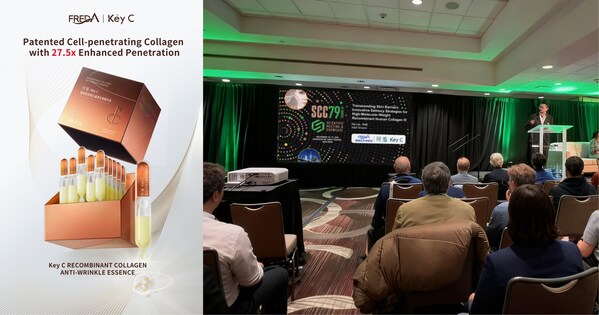
Freda Key C Presents Advanced Recombinant Collagen Delivery Technology at SCC79
Novel Delivery System for High-Molecular-Weight Collagen Advances Anti-Aging Science at the Society of Cosmetic Chemists' Annual Meeting NEW YORK, Dec. 18, 2025 /PRNewswire/ -- Shandong Freda Biotech Co., Ltd. and its medical-aesthetic skincare brand Key C announced the presentation of a novel c...
Global study targets dengue as disease threatens nearly half the world's population
LISMORE, Australia, Dec. 18, 2025 /PRNewswire/ -- Thousands of dengue forecasting models have been published, but few have been tested in real public-health settings. Now, researchers from the US andAustralia are launching a field evaluation inVietnam to see whether a new early-warning platform c...
EPS Corporation Selects ArisGlobal's LifeSphere® MultiVigilance
Prominent Japan CRO adopts new safety case processing system to further enhance
compliance, automation, and efficiency
BOSTON, Dec. 18, 2025 /PRNewswire/ -- ArisGloba
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 319 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 318 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 277 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 256 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 250 media titles]
2026-02-16 08:00