Pharmaceuticals
CSL Vifor and Travere Therapeutics Announce Swissmedic approval of FILSPARI® (sparsentan) for the treatment of IgA Nephropathy
Temporary marketing authorization is based on statistically significant and clinically meaningful results from the phase-III PROTECT trial ST. GALLEN, Switzerland and SAN DIEGO, Oct. 17, 2024 /PRNewswire/ -- CSL Vifor and Travere Therapeutics, Inc., (NASDAQ: TVTX) today announced that Swissmedic...
IND Application for a Phase Ⅲ Clinical Study of KN026 Combined with Albumin-bound Docetaxel as Neoadjuvant Treatment for Breast Cancer was Approved
SUZHOU, China, Oct. 17, 2024 /PRNewswire/ -- Alphamab Oncology (stock code: 9966 HK) and CSPC Pharmaceutical Group Co., Ltd. ("CSPC") (stock code: 1093.HK) jointly announced that an application for a phase III clinical study (Study ID: KN026-004) of the anti-HER2 bispecific antibody KN026 combine...
Innovent Announces Phase 2 Clinical Study of Picankibart (IBI112) in Chinese Patients with Ulcerative Colitis Met Primary Endpoint
SAN FRANCISCO and SUZHOU, China, Oct. 17, 2024 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, cardiovascular and metabo...
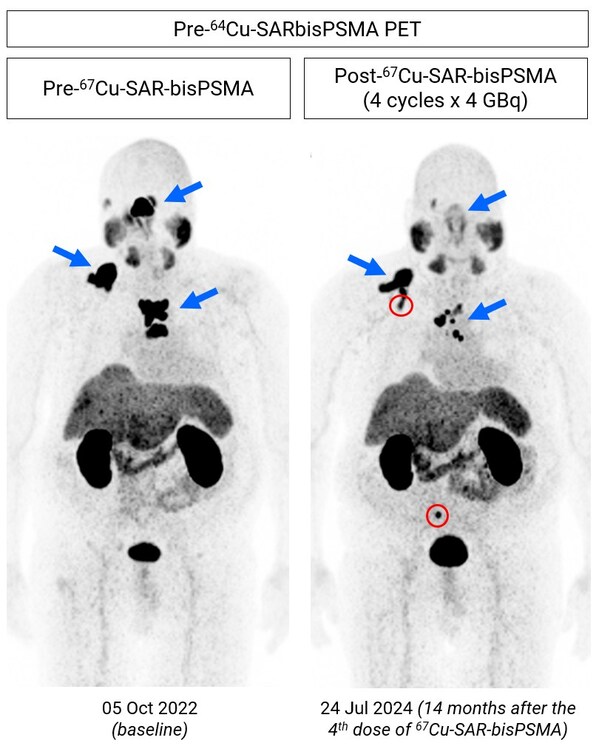
Copper-67 SAR-bisPSMA updates
SYDNEY, Oct. 16, 2024 /PRNewswire/ -- HIGHLIGHTS Cohort 4 - SECuRE Trial * The third participant of cohort 4 (multi-dose) of the SECuRE trial1 has now completed the Dose Limiting Toxicity (DLT) period after a second dose of 12GBq of67Cu-SAR-bisPSMA, following on from the announcement dated 1...
IGCS Late Breaking Abstract and The Lancet: Akeso Published Positive PFS and OS Results from Phase 3 First-Line Study of Cadonilimab in Cervical Cancer
HONG KONG, Oct. 16, 2024 /PRNewswire/ -- Akeso Biopharma (9926.HK) (" Akeso", the "Company" ) announced positive results in progression-free survival (PFS) and overall survival (OS) from its Phase 3 clinical study (COMPASSION-16/AK104-303). This study evaluated the efficacy of its independently...
TenNor Announces More than 300 Million RMB Financing to Support Development and Commercialization of Late-Stage Assets Including Rifasutenizol for Heliobacter pylori Infections
SUZHOU, China, Oct. 16, 2024 /PRNewswire/ -- TenNor Therapeutics, a clinical stage company dedicated to developing new therapies to address unmet needs in infectious diseases, announced today the initial closing of a Series E financing round for more than300 million RMB. New investor AMR Action F...
SONIRE's HIFU Therapy System Designated as Breakthrough Device by FDA
TOKYO, Oct. 16, 2024 /PRNewswire/ -- SONIRE Therapeutics Inc. (hereinafter referred to as "SONIRE"), based inTokyo, Japan, announces that its self-developed next-generation HIFU (High-Intensity Focused Ultrasound) therapy system (development code: Suizenji) has been designated as a breakthrough ...
Frost & Sullivan grants the 2024 Global Pluripotent stem cell drug R&D Innovation Award to ZEPHYRM BIOTECHNOLOGIES
SHANGHAI, Oct. 16, 2024 /PRNewswire/ -- The 18th The Growth Innovation Leadership (GIL) Council & the 3rd New Investment Conference in 2024 was held in Shanghai from August 28 to 30, 2024. On the evening of August 28, the prestigious 2024 Global and China Awards for Growth, Innovation, and Leaders...
PharmAust affirms corporate strategy with name change to Neurizon Therapeutics
MELBOURNE, Australia, Oct. 15, 2024 /PRNewswire/ -- Neurizon Therapeutics Limited (ASX: NUZ) ("Neurizon" or "the Company"), a clinical-stage biotech company dedicated to advancing treatments for neurodegenerative diseases, is pleased to announce it has officially changed its name from PharmAust L...
Chiglitazar for the Treatment of MASH Phase II Clinical Study Selected for Oral Presentation at the 2024 American Liver Disease Annual Meeting
SHENZHEN, China, Oct. 15, 2024 /PRNewswire/ -- On October 15, 2024, the list of selected candidates for the oral report of the American Association for the Study of the Liver (AASLD 2024) annual meeting was officially announced online. The abstract of the Phase II clinical study (CGZ203 trial) on...
Bloomage Debuts New Products at CPHI Milan to Reinforce Its Commitment to Innovation
MILAN, Oct. 15, 2024 /PRNewswire/ -- Bloomage, a global leader in hyaluronic acid and other bioactive substance innovations, unveiled its Hyatrue® Sterile Sodium Hyaluronate (HA) and showcased its BloomseaN™ Polydeoxyribonucleotide (PDRN) and Polynucleotide (PN) at CPHI Milan. Known to be at "the...
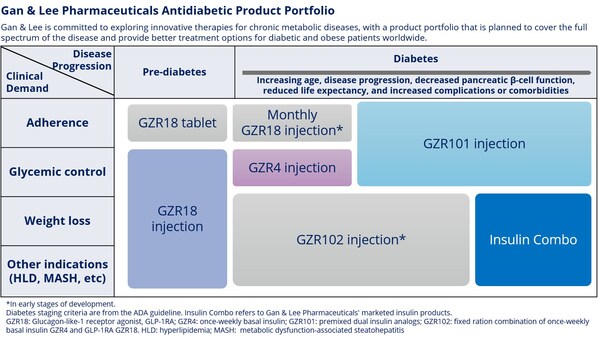
Gan & Lee Pharmaceuticals' Three Innovative Drugs: GZR18 Injection, GZR4 Injection, and GZR101 Injection Achieve Primary Endpoints in Phase 2 Clinical Studies
* After 24 weeks of treatment in patients with Type 2 diabetes, the bi-weekly GLP-1 receptor agonist GZR18 injection showed superior efficacy in lowering HbA1c and body weight compared to semaglutide (Ozempic®). * After 16 weeks of treatment in patients with Type 2 diabetes, the once-weekly i...
PharmAbcine Announces Safety Approval for 4mg Single-dose Cohort in Phase 1 Clinical Trial of PMC-403 for Neovascular Age-related Macular Degeneration
* Safety Review Committee (SRC) approves single ascending dose 4mg cohort in Phase 1 trial of PMC-403, preparing for multiple ascending dose 4 mg cohort. * PMC-403 is being explored in broader therapeutic areas, including rare vascular disease systemic capillary leak syndrome (SCLS) and kidney...
Positive guidance from the U.S. FDA on 64Cu-SAR-bisPSMA Phase III trial in patients with recurrence of prostate cancer
Highlights * United States Food and Drug Administration (U.S. FDA) provided positive feedback on a pivotal Phase III trial for64Cu-SAR-bisPSMA diagnostic in prostate cancer patients with biochemical recurrence (BCR), AMPLIFY. * The positive results of the completed COBRA and PROPELLER trials,...
Tsingke Shines at Cell & Gene Therapy International 2024
BEIJING and BOSTON, Oct. 14, 2024 /PRNewswire/ -- Beijing Tsingke Biotech Co., Ltd. recently concluded a successful exhibition at CGTI2024 inBoston. This premier event attracted over 3,200 global attendees, including professionals from the top 20 biopharma companies. Tsingke showcased its innovat...
US FDA issues Study May Proceed letter for the Pilot Study of Pidnarulex Pharmacodynamics in Patients with Advanced Solid Tumors, Sponsored by the US National Cancer Institute
TAIPEI and SAN DIEGO, Oct. 14, 2024 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and infectious diseases, today announced that FDA issues Study May Proceed letter for its developing drug Pid...
Samsung Biologics launches high-concentration formulation platform to accelerate high-dose drug development
* S-HiConTM is designed to maximize drug delivery and stability through high-concentration formulation * The platform can address challenges associated with viscosity and achieve stable liquid formulation for over 200 mg/mL subcutaneous administration INCHEON, South Korea, Oct. 14, 2024 /PRNe...
BioCity will present the late-breaking clinical trial data of its ETA selective antagonist SC0062 at the American Society of Nephrology (ASN) 2024 with simultaneous publication of the trial data in the Journal of the American Society of Nephrology (JASN)
SHANGHAI, Oct. 14, 2024 /PRNewswire/ -- BioCity Biopharma announced that a late-breaking clinical trial abstract of its endothelin receptor type A (ETA) selective antagonist SC0062 has been selected for oral presentation at ASN Kidney Week 2024 which will take place fromOctober 23 to 27 in San Di...
Akeso Secures $250 Million USD to Propel Global Expansion of Its Innovative Drug Pipeline
Expediting International Clinical Trials for Pivotal Products Key Participants Include International Long-Term and Healthcare Funds HONG KONG, Oct. 13, 2024 /PRNewswire/ -- Akeso Biopharma (9926. HK) ("Akeso", the "Company" ) announced that it has successfully raised approximately$250 million US...
Delegation of International Communication Experts and Foreign Media Outlets Visits Emerging Industry Enterprises in Zhongshan, Guangdong Province
BEIJING, Oct. 11, 2024 /PRNewswire/ -- On October 9, 2024, the delegation of international communication experts and foreign media outlets, themed "The World Comes to Zhongshan" and organized by the CICG Academy of Translation and Interpretation, visited several representative enterprises in Zhon...
Week's Top Stories
Most Reposted
Agoda Launches Agoda Impact Lab at ASEAN Tourism Forum
[Picked up by 322 media titles]
2026-01-29 15:06Wonder Raises USD 12 Million Venture Debt from HSBC Innovation Banking to Drive Growth and Expansion
[Picked up by 322 media titles]
2026-02-02 10:00AI adoption is widespread, but developer confidence is still catching up, Agoda report finds
[Picked up by 312 media titles]
2026-02-03 11:00Colebrook Bosson Saunders Officially Launches Lana, A Circular Ergonomic Laptop Stand for the Hybrid Generation
[Picked up by 303 media titles]
2026-02-03 12:00Singapore Airshow 2026 Milestone Edition: 20 Years of Shaping the Aerospace Landscape as Asia-Pacific Drives Global Growth
[Picked up by 286 media titles]
2026-02-01 19:35