Pharmaceuticals
Alphamab Oncology Announces Biparatopic HER2-targeting ADC JSKN003 Was Granted Breakthrough Therapy Designation by the FDA for the Treatment of PROC
SUZHOU, China, Dec. 20, 2025 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the biparatopic HER2-targeting antibody-drug conjugate (ADC) JSKN003, independently developed by the Company, and co-developed with JMT-Bio Technology Co., Ltd., a wholly-owned subsidiary of CSPC P...
WuXi Biologics Achieves CDP Highest "A" Ratings in Both Climate Change and Water Security
SHANGHAI, Dec. 19, 2025 /PRNewswire/ -- WuXi Biologics (2269.HK) announced that it has been named to the CDP "A" lists for both Climate Change and Water Security for 2025, underscoring its leadership in environmental stewardship and transparent disclosure. Climate Change Leadership Being recogn...
NEW DRUG APPLICATION FOR TINENGOTINIB TABLETS ACCEPTED BY THE NATIONAL MEDICAL PRODUCTS ADMINISTRATION
NANJING, China and GAITHERSBURG, Md., Dec. 18, 2025 /PRNewswire/ -- TransThera Sciences Nanjing, Inc. (the "TransThera") announced that the new drug application for Tinengotinib tablets has been accepted by the Center for Drug Evaluation ("CDE")of the National Medical Products Administration ("NM...
Neuraxpharm Goes Live with TraceLink MINT to Standardize Real‑Time Data Exchange Across Its Global Supply Chain Network
The European specialty pharmaceutical company establishes a unified digital data foundation to enable future agentic orchestration BOSTON, Dec. 18, 2025 /PRNewswire/ -- TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the supply chain, today announced t...
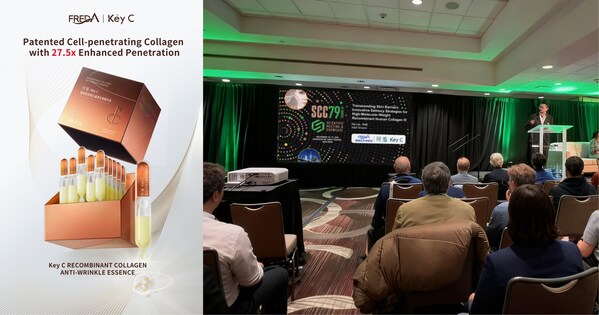
Freda Key C Presents Advanced Recombinant Collagen Delivery Technology at SCC79
Novel Delivery System for High-Molecular-Weight Collagen Advances Anti-Aging Science at the Society of Cosmetic Chemists' Annual Meeting NEW YORK, Dec. 18, 2025 /PRNewswire/ -- Shandong Freda Biotech Co., Ltd. and its medical-aesthetic skincare brand Key C announced the presentation of a novel c...
EPS Corporation Selects ArisGlobal's LifeSphere® MultiVigilance
Prominent Japan CRO adopts new safety case processing system to further enhance
compliance, automation, and efficiency
BOSTON, Dec. 18, 2025 /PRNewswire/ -- ArisGloba
Alphamab Oncology Announces IND Application for Innovative PD-L1/ VEGFR2 Bispecific ADC JSKN027 was Officially Accepted by CDE
SUZHOU, China, Dec. 18, 2025 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that the Investigational New Drug (IND) application for JSKN027, an independently developed innovative bispecific antibody-drug conjugate (ADC) targeting PD-L1 and VEGFR2, has been officially accepted b...
Nature | Two Phase 3 Clinical Results of Mazdutide (GLP-1/GCG Dual Receptor Agonist) in Chinese Adults with Type 2 Diabetes Have Been Back-to-Back Published in Nature
SAN FRANCISCO and SUZHOU, China, Dec. 17, 2025 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncologic, autoimmune, cardiovascular and metaboli...
PRISM BioLab and Talus Bioscience Join Forces to Discover Novel Inhibitors of Transcription Factor and Protein-Protein Interaction Targets
TOKYO, Dec. 17, 2025 /PRNewswire/ -- PRISM BioLab, Co. Ltd. ("PRISM") and Talus Bioscience, Inc. ("Talus Bio") announced today that they entered into a collaboration to discover novel Inhibitors of transcription factor (TF) and protein-protein interaction (PPI) targets. By combining Talus Bio's a...
Rona Therapeutics Unveils the Global First Bi-valent PCSK9-LPA siRNA into Clinical Development for Cardiovascular Risk Reduction
SHANGHAI and SANTA BARBARA, Calif., Dec. 17, 2025 /PRNewswire/ -- Rona Therapeutics, a global leader in next-generation RNAi medicines, today announced the submission ofRN5681 to the Australian Human Research Ethics Committee (HREC), advancing the company's first bi-valent siRNA into clinical de...
MEDI&GENE Announces Catalyze Agreement with Lilly to Advance Next-Generation Obesity Therapeutics
SEOUL, South Korea, Dec. 17, 2025 /PRNewswire/ -- MEDI&GENE, a
biopharmaceutical company developing therapeutics for metabolic diseases,
announced today that it has entered into a Catalyze agreement with
Eli Lilly and Company (Lilly) to advance a next-generation therapeutic for
obesity.
Everest Medicines Announces that Licensing Partner LIB Therapeutics has Received U.S. FDA Approval of LEROCHOL™ (lerodalcibep-liga) for Adults with Elevated LDL Cholesterol
SHANGHAI, Dec. 17, 2025 /PRNewswire/ -- Everest Medicines (HKEX 1952.HK, "Everest", or the "Company"), a biopharmaceutical company focused on the discovery, clinical development, manufacturing, and commercialization of innovative therapeutics, today announced that the U.S. Food and Drug Administ...
111, Inc. Announces Third Quarter 2025 Unaudited Financial Results
* Transition from An Asset-Heavy Business Model to An Asset-Light Business Model * Achieved Quarterly Non-GAAP Net Profitability * Maintained Non-GAAP Operational Profitability for Three Consecutive Quarters * Achieved Quarterly Positive Operating Cash Flow SHANGHAI, Dec. 17, 2025 /PRNewswi...
Antengene Expands XPOVIO® Indications in Malaysia with Approval in Diffuse Large B-cell Lymphoma
SHANGHAI and HONG KONG, Dec. 17, 2025 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative, commercial-stage global biotech company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for autoimmune dis...
Jiaying Pharmaceutical: A New Pathway for the Industrialization of Century-Old Hakka Medicine
HONG KONG, Dec. 17, 2025 /PRNewswire/ -- Recently, Meizhou in Guangdong, known as the "World Capital of the Hakka", welcomed a globally watched event, the Seventh World Hakka Entrepreneurs Convention. At this gathering that drew the attention of Hakka entrepreneurs worldwide, Guangdong Jiaying Ph...
Allink Biotherapeutics Completes $47M Extension Rounds of Series A to Accelerate Clinical Programs and Novel Platforms Development
* The company secured $47 million through extension rounds of Series A led by Legend Capital and Meituan Long-Z Investment with strong backing from both new investors and existing supporters * Proceeds to advance two differentiated and highly competitive ADC programs in global Phase I trials ...
Breakthrough Progress: METiS TechBio Publishes Consecutive Research Findings in Nature Communications and the Journal for ImmunoTherapy of Cancer
BEIJING, Dec. 16, 2025 /PRNewswire/ -- METiS TechBio ("METiS") today announced that two of its oncology pipeline candidates,MTS-105 and MTS-107, have been published in leading international peer-reviewed journals,Nature Communications and theJournal for ImmunoTherapy of Cancer (JITC), representin...
Breaking the "Untreatable": Biostar Pharma's UTD1 Achieves First Patient Dosing in U.S. for Pivotal Clinical Trial for Breast Cancer Brain Metastases
SAN FRANCISCO, Dec. 15, 2025 /PRNewswire/ -- Biostar Pharma, Inc., the U.S. wholly-owned subsidiary of Beijing Biostar Pharmaceuticals Co., Ltd. (Stock Cod e: 2563.HK), today announced that the first patient has been dosed for one of its key oversea clinical studies: the U.S. pivotal clinical stud...
HitGen Submits Commitment Letter to Science Based Targets initiative (SBTi)
CHENGDU, China, Dec. 15, 2025 /PRNewswire/ -- HitGen Inc. (hereinafter referred to as "HitGen", SSE: 688222.SH), announced it has officially submitted its commitment letter to the Science Based Targets initiative (SBTi). HitGen has pledged to set near-term science-based targets within the next tw...
Rona Therapeutics Advances INHBE siRNA Into Phase 1 Clinical Development
SHANGHAI and SANTA BARBARA, Calif., Dec. 15, 2025 /PRNewswire/ -- Rona Therapeutics, a clinical-stage RNAi company, today announced the recent completion of Cohort 1 dosing in its Phase 1 first-in-human clinical study of RN3161, an investigational GalNAc-conjugated siRNA targeting INHBE for obesit...
Week's Top Stories
Most Reposted
DBS is First Bank in Asia Pacific to Pilot Visa Intelligent Commerce for Everyday Payments
[Picked up by 320 media titles]
2026-02-16 10:00Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 319 media titles]
2026-02-19 14:30Little Artists Art Studio, Singapore Shines at Art Capital 2026
[Picked up by 278 media titles]
2026-02-17 19:12Kung Fu Meets Spring -- Unitree Spring Festival Gala Robots Present "Cyber Real Kung Fu" in the Year of the Horse
[Picked up by 257 media titles]
2026-02-17 14:16SMU MBA Rises in FT Global Rankings, Excelling in ESG, Salary and Value-for-Money
[Picked up by 251 media titles]
2026-02-16 08:00