Pharmaceuticals
Kintor Pharma Announced Successful Dosing of the First Patient for Acne Vulgaris Phase II Clinical Trial of KX-826
SUZHOU, China, Jan. 24, 2022 /PRNewswire/ -- Kintor Pharmaceutical Limited ("Kintor Pharma," HKEX: 9939), a clinical-stage biotechnology company developing innovative small molecules and biological therapeutics, today announced the first patient dosing in its phase II clinical trial of KX-826("p...
Samsung Biologics Reports Fourth Quarter & Fiscal Year 2021 Financial Results
* Q4'21 revenue of KRW 444.3 billion increased 18% compared to Q4'20. * Q4'21 operating profit of KRW 128.8 billion increased 39% compared to Q4'20. * FY'21 revenue of KRW 1,568.0 billion, an increase of 35% compared to FY'20. * FY'21 operating profit of KRW 537.3 billion, an increase of 84% ...
Harbour BioMed Announces Dosing of First Patient in Combination Therapy Phase Ib/IIa Trial of Next-Generation Anti-CTLA-4 Antibody
CAMBRIDGE, Mass. and ROTTERDAM, Netherlands and SUZHOU, China, Jan. 24, 2022 /PRNewswire/ -- Harbour BioMed ("HBM", HKEX: 02142) announced that, it has successfully completed the dosing of first patient in phase Ib/IIa trial at the stage of dose expansion of its anti-CTLA-4 antibody (HBM4003) in ...
Innovent Announces the Approval of Pemazyre® (pemigatinib) in Hong Kong Market for the Treatment of Adults With Locally Advanced Or Metastatic Cholangiocarcinoma With A FGFR2 Fusion Or Rearrangement That Have Progressed After At Least One Prior Line Of Systemic Therapy
SAN FRANCISCO and SUZHOU, China, Jan. 24, 2022 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseas...
Applied Pharmaceutical Science Inc. Announces FDA Approval of Investigational New Drug Application for APS03118, a Next generation RET Original New Drug for Unlimited Cancers
BEIJING and PHILADELPHIA, Jan. 24, 2022 /PRNewswire/ -- Applied Pharmaceutical Science, Inc. ("APS" or "the Company"), has recently announced the Investigational New Drug (IND) application for its self-developed breakthrough new drug APS03118, a next generation selective RET inhibitor, has been a...
Ascletis Announces First Patient Dosed in the Phase III Clinical Trial of FASN Inhibitor ASC40 Combined with Bevacizumab for Treatment of Recurrent Glioblastoma
HANGZHOU and SHAOXING, China, Jan. 23, 2022 /PRNewswire/ -- Ascletis Pharma Inc. (HKEX code: 1672) today announces the dosing of the first patient in the Phase III registration clinical trial of ASC40 combined with bevacizumab for treatment of recurrent glioblastoma (rGBM). ASC40 is an oral, sele...
Innovent and Eli Lilly and Company Announced Final Clinical Results and Biomarker Analysis of Phase Ib Study of TYVYT® (Sintilimab Injection) Plus Bevacizumab Biosimilar Injection for Advanced Hepatocellular Carcinoma at ASCO GI Annual Meeting 2022
SAN FRANCISCO and SUZHOU, China, Jan. 22, 2022 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent", HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major disea...
Swiss Pharma Company - IDEOGEN takes over Special Access Program for BELEODAQ® (belinostat)
HURDEN, Switzerland, Jan. 22, 2022 /PRNewswire/ -- IDEOGEN GROUP, Managed Access division, takes over Special Access Program for BELEODAQ® (belinostat) in Europe, Middle East, North Africa, Russia, and CIS. BELEODAQ® is a prescription medicine used to treat patients with a rare form of blood canc...
Sihuan Pharmaceutical (0460.HK): Establishment of Joint Venture with Bluepha Co., Ltd.
HONG KONG, Jan. 21, 2022 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Co., Ltd. (the "Company" or "Sihuan Pharmaceutical", together with its subsidiaries, collectively referred to as the "Group:" Hong Kong Stock Exchange Stock Code: 0460) is pleased to announce thatBeijing MeiYan KongJian...
Hinova Announces First Patient Dosed in a Phase I Clinical Trial of HP518, an orally bioavailable Chimeric Degrader targeting Androgen Receptor for Prostate Cancer Treatment
MELBOURNE, Australia, Jan. 21, 2022 /PRNewswire/ -- Hinova Pharmaceuticals Inc., a clinical-stage biopharmaceutical company focused on developing novel therapeutics for cancers and metabolic diseases through targeted protein degradation technologies, announced that the first patient with metastat...
HanAll Biopharma Reports Full-Year 2021 Results and Provides Business Update
* 2021 sales of KRW 101.6 billion for a 15% increase from 2020 * 2021 operating profit of KRW 10.1 billion grew by 70% from 2020 * Increasing milestone payments and revenues from R&D activities are reinvested into new R&D programs SEOUL, South Korea, Jan. 20, 2022 /PRNewswire/ -- [Busines...
Bridget Johnson Joins CSafe Global as Senior Vice President of Marketing
CSafe Global makes strategic addition to its senior leadership team bringing Bridget Johnson on board as the SVP of Marketing DAYTON, Ohio, Jan. 20, 2022 /PRNewswire/ -- CSafe Global, the innovation leader in temperature-controlled container solutions for the transport of life-enhancing pharma...
Victor Papamoniodis Joins Medison Pharma as VP International Markets
ZUG, Switzerland, Jan. 20, 2022 /PRNewswire/ -- Medison
Hinova Announces First Patient Dosed in a Phase I Clinical Trial of HP518, an Orally Bioavailable Chimeric Degrader Targeting Androgen Receptor for Prostate Cancer Treatment
MELBOURNE, Australia, Jan. 20, 2022 /PRNewswire/ -- Hinova Pharmaceuticals Inc., a clinical-stage biopharmaceutical company focused on developing novel therapeutics for cancers and metabolic diseases through targeted protein degradation technologies, today announced that the first patient with m...
Senhwa's Silmitasertib Receives US FDA Orphan Drug Designation for the Treatment of Biliary Tract Cancer
TAIPEI and SAN DIEGO, Jan. 20, 2022 /PRNewswire/ -- Senhwa Biosciences, Inc. (TPEx: 6492), a drug development company focusing on first-in-class therapeutics for oncology, rare diseases, and novel coronaviruses, announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Des...
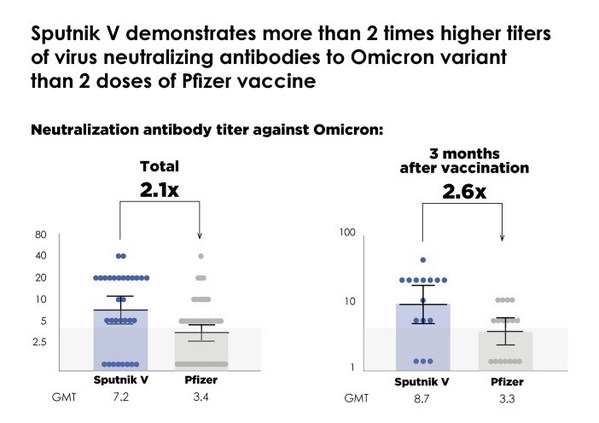
Sputnik V demonstrates strong protection against Omicron variant, with over 2 times higher virus neutralizing activity compared to the Pfizer vaccine according to a unique independent comparative study conducted by the Spallanzani Institute in Italy
Previous studies also demonstrated additional significant strengthening of protection against Omicron by Sputnik Light booster, which can also be a universal booster to other vaccines to strengthen and lengthen their protection against Omicron. MOSCOW, Jan. 20, 2022 /PRNewswire/ -- * A uniqu...
Successful Completion of First Patient Implantation of MINI WELL® and MINI WELL TORIC® Extended Depth-of-Focus IOLs in China Announced by Affamed Technologies, Joint Venture between AffaMed Therapeutics and SIFI
CATANIA, Italy, Jan. 20, 2022 /PRNewswire/ -- SIFI, a leading international ophthalmic company headquartered inItaly, is pleased to divulge the successful completion of the first patient implantation of MINI WELL® and MINI WELL TORIC® IOLs inChina, announced via Affamed Technologies, Joint Ventur...
Glenmark Specialty S.A. and Lotus International Pte. Ltd. enter into Exclusive Licensing Agreement for commercializing Ryaltris (TM) Nasal Spray in Singapore, Hong Kong and Vietnam
* Under the terms of the agreement, Glenmark will be responsible for the manufacture and supply of Ryaltris™, while Lotus International Pte. Ltd. will be responsible for the commercialization of Ryaltris™ (subject to receipt of regulatory approvals) across these markets. * Glenmark will recei...
Nippon Express (Belgium) Acquires GDP Certification for Facility in Brussels Airport's Cargo Area
TOKYO, Jan. 20, 2022 /PRNewswire/ -- Nippon Express (Belgium) N.V./S.A. (hereinafter "NX Belgium"), a company of the Nippon Express Holdings, Inc. Group, has obtained Good Distribution Practice (GDP) certification, effective December 21, 2021, for air and ground forwarding operations, inclusive of...
Award: Boehringer Ingelheim is Global Top Employer 2022
* Boehringer Ingelheim advances to one of eleven global top employers worldwide * This is the second consecutive year for the global certification, and the fifth consecutive year for the certification inSingapore * High scores in values, ethics and integrity serve as a driver for a strong c...
Week's Top Stories
Most Reposted
LONG AN INTERNATIONAL PORT JOINS 12TH PORTECH ASIA SUMMIT 2025 IN MALAYSIA
[Picked up by 332 media titles]
2025-01-18 03:30Hong Kong Airlines Takes Off to Australia's Gold Coast Bringing Popular Travel Option for the Chinese New Year
[Picked up by 306 media titles]
2025-01-18 17:00HARD ROCK HOTEL BALI ACHIEVES PRESTIGIOUS GSTC CERTIFICATION
[Picked up by 296 media titles]
2025-01-20 14:27RuggON Launches VIKING II: Transforming Fleet Management with Unmatched Technology and Flexibility
[Picked up by 295 media titles]
2025-01-14 21:00ARK Wealth Black and Diamond Client Summit: Enhancing Wealth Allocation Strategies for a New Era
[Picked up by 281 media titles]
2025-01-17 13:37