Pharmaceuticals
Ascentage Pharma's MDM2-p53 Inhibitor Alrizomadlin (APG-115) Granted Fast Track Designation by the US FDA for the Treatment of Relapsed/Refractory Unresectable or Metastatic Melanoma
SUZHOU, China, and ROCKVILLE, MD, Sept 22, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a global biopharmaceutical company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that its novel MDM2-p53 inhibitor alrizomadlin (A...
AbCellera and Everest Medicines Announce Multi-Target Collaboration to Advance New Antibody Therapies
VANCOUVER, British Columbia and SHANGHAI, Sept. 23, 2021 /PRNewswire/ --
AbCellera
INOVIO Receives Regulatory Authorization to Conduct Phase 3 Efficacy Trial of its COVID-19 DNA Vaccine Candidate, INO-4800, in Mexico
News follows recent regulatory authorizations to proceed with the trial in Brazil and the Philippines INOVIO and partner Advaccine are collaborating on the global Phase 3 trial in regions underserved by COVID-19 vaccines; to focus onLatin America, Asia, and Africa PLYMOUTH MEETING, Pa., Sept. 2...
Clover's COVID-19 Vaccine Candidate Demonstrates 79% Efficacy Against Delta in Global Phase 2/3 SPECTRA Trial Dominated by Variants of Concern and Interest
* Trial enrolled over 30,000 adult & elderly participants across 4 continents; 100% of SARS-CoV-2 strains observed in efficacy analysis were variants (Delta was predominant strain) * Primary and secondary efficacy endpoints were successfully met * 100% efficacy against severe COVID-19 & hosp...
Significantly Improved Safety Profile and Metabolism of Remdesivir Observed Due to Encapsulation in NanoViricides Drug Candidate Enabling Potential Highly Effective Pan-Coronavirus Antiviral Drug
SHELTON, Conn., Sept. 22, 2021 /PRNewswire/ -- NanoViricides, Inc. (NYSE American: NNVC) (the "Company"), a leader in the development of highly effective antiviral therapies based on a novel nanomedicines technology, reported today on the significant advantages gained by remdesivir encapsulation ...
Samsung Biologics showcases its newest CDO process platform, S-Cellerate™, at BPI 2021, offering expedited timeline to IND and BLA
INCHEON, South Korea, Sept. 22, 2021 /PRNewswire/ -- Samsung Biologics, a global contract development and manufacturing organization (CDMO), introduced its proprietary technology platform, S-CellerateTM, at the BioProcess International Conference (BPI 2021) to offer clients an integrated and seam...
New Therapy to Treat Advanced Small Cell Lung Cancer ZEPZELCA® (lurbinectedin) Approved in Singapore
Highlights: * ZEPZELCA® (lurbinectedin) is the first new therapy approved in Singapore to treat 2L metastatic small cell lung cancer (SCLC) in 20 years * ZEPZELCA provisional approval represents an important advance for adult patients whose metastatic SCLC has progressed on or after platinum-...
Koji Tanabe, CEO of I Peace, Inc. honored by World Biz Magazine with "Top 100 Innovation CEO" Award
PALO ALTO, Calif., Sept. 21, 2021 /PRNewswire/ -- Koji Tanabe, Founder and CEO
of I Peace, Inc. (https://www.ipeace.com
FDA Approves LEXETTE (R) for Adolescent Plaque Psoriasis
RALEIGH, N.C., Sept. 21, 2021 /PRNewswire/ -- Mayne Pharma is pleased to announce that the U.S. Food and Drug Administration (FDA) has approved LEXETTE® (halobetasol propionate) foam, 0.05% for use in adolescents. LEXETTE, a super potent topical corticosteroid, is now approved for the treatment ...
Kyowa Hakko Acclaimed by Frost & Sullivan for its Non-GMO, Allergen-free, Immune Support Paraprobiotic Ingredient, IMMUSE™
The solution offers superior ease of formulation, potential application versatility, and more comprehensive immune support compared to probiotics and other immune health ingredients SANTA CLARA, Calif., Sept. 21, 2021 /PRNewswire/ -- Based on its recent analysis of the North American immune hea...
Alterity Announces Presentation of Biomarker Data at the International Parkinson and Movement Disorder Society Congress 2021
MELBOURNE, Australia and SAN FRANCISCO, Sept. 20, 2021 /PRNewswire/ -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) ("Alterity" or "the Company"), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative conditions, today announced that data was present...
CStone presents clinical data from the China registrational bridging study on ivosidenib in patients with relapsed/refractory acute myeloid leukemia (R/R AML) with a susceptible IDH1 mutation at the 2021 ESMO Congress
* The data showed that ivosidenib demonstrated clinical efficacy in the treatment of Chinese adults with R/R AML with a susceptible IDH1 mutation. And ivosidenib was well tolerated and had a manageable safety profile * In August 2021, the National Medical Products Administration (NMPA) of Chin...
Bionomics Prepares BNC210 for Start of Phase 2 Acute Treatment of Social Anxiety Disorder Trial
* Rapid oral absorption of BNC210 novel tablet formulation potentially well-suited for acute treatment of anxiety in patients with Social Anxiety Disorder * Phase 2 clinical trial on target to start by end of 2021 and expected to read out topline data by end of 2022 ADELAIDE, Australia, Sept....
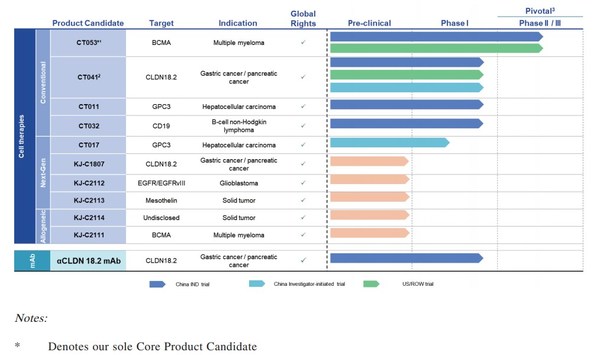
Appealing Data of CARsgen Therapeutics' CAR-T (CT041) in Advanced Gastric Cancer Presented at ESMO
SHANGHAI, Sept. 19, 2021 /PRNewswire/ -- On September 19, 2021, CARsgen Therapeutics (stock code: 2171.HK) disclosed the latest progress of the investigator-initiated trial (IIT) of Claudin18.2 (CLDN18.2) CAR-T (CT041) for the treatment of digestive system tumors. Results of this trial have been ...
CStone presents preliminary results from a phase Ib study of the anti-CTLA-4 monoclonal antibody (mAb) CS1002 in combination with the anti-PD-1 mAb CS1003 in patients with advanced solid tumors at ESMO 2021
SUZHOU, China, Sept. 19, 2021 /PRNewswire/ -- CStone Pharmaceuticals ("CStone", HKEX: 2616), a leading biopharmaceutical company focused on the research, development, and commercialization of innovative cancer immunotherapies and precision medicines, today announced the preliminary results from t...
2021 CSCO | Henlius will Release Four Oral Presentations of Two Upcoming Commercial Products: Novel anti-PD-1 mAb Serplulimab and Bevacizumab Biosimilar
SHANGHAI, Sept. 18, 2021 /PRNewswire/ -- The 24th Annual Meeting of Chinese Society of Clinical Oncology (CSCO) will be held online and in-person from 25th to 29th September 2021. In this meeting, Henlius will release 4 study results of 2 products to be commercialized, the novel anti-PD-1 mAb ser...
Innovent Releases Interim Analysis Results of Sintilimab in Combination with Chemotherapy for the First-Line Treatment of Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma in the Phase 3 ORIENT-16 Study at ESMO Congress 2021
SAN FRANCISCO and SUZHOU, China, Sept. 18, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent", HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major dise...
Innovent Releases Interim Analysis Results of Sintilimab plus Chemotherapy for the First-Line Treatment of Esophageal Squamous Cell Carcinoma in the Phase 3 ORIENT-15 Study at ESMO Congress 2021
SAN FRANCISCO and SUZHOU, China, Sept. 18, 2021 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high quality medicines for the treatment of cancer, metabolic, autoimmune and other major dis...
InnoCare and Keymed Jointly Announce Approval of Clinical Trial of CD20xCD3 Bispecific Antibody CM355
BEIJING, Sept. 17, 2021 /PRNewswire/ -- InnoCare Pharma (HKEX: 09969) and Keymed Biosciences (HKEX: 02162) jointly announced today that the Investigational New Drug (IND) of CM355, a CD20xCD3 bispecific antibody developed by a joint venture between the two companies called Tiannuojiancheng Pharm...
The NDA of Henlius Novel Anti-PD-1 mAb Serplulimab for First-Line Treatment of sqNSCLC Accepted by China's NMPA, Phase 3 MRCT Met its Primary Endpoint
SHANGHAI, Sept. 17, 2021 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696.HK) announced that the New Drug Application (NDA) of Serplulimab Injection (HLX10), a novel anti-PD-1 monoclonal antibody (mAb) independently developed by the company, in combination with carboplatin and albumin-bound ...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00Announcement of "Cyber Sousa Award" Winners of the 16th Xiamen International Animation Festival
[Picked up by 272 media titles]
2024-11-26 16:48