Pharmaceuticals
New 3M™ Harvest RC clarifier brings chromatography technology to harvest clarification with a single-stage solution for upstream processes
ST. PAUL, Minn., June 2, 2021 /PRNewswire/ -- 3M Health Care launched 3M™ Harvest RC clarifier, leveraging its proprietary fibrous chromatography media to deliver a single-stage purification solution for recombinant protein therapeutic manufacturing. It is the next generation in harvest and clar...
Daewoong Pharmaceutical Publishes Results of First Clinical Study of SGLT-2 Inhibitor 'Enavogliflozin' for Canine Diabetes
SEOUL, South Korea, June 1, 2021 /PRNewswire/ -- Daewoong Pharmaceutical (CEO Sengho Jeon) announced that the results of an investigator-initiated clinical study on the effect of Enavogliflozin, originally developed as Type II Diabetes Treatment forhumans, on treating canine diabetes were publish...
GenScript ProBio and Neoletix signed a letter of intent on clinical and commercial production of recombinant human coagulation factor VIII
NANJING, China, June 1, 2021 /PRNewswire/ -- On June 1st 2021, GenScript ProBio and Neoletix (Beijing) Biotechnology Co., Ltd. (hereinafter referred to as Neoletix) signed a letter of intent on cooperation for clinical and commercial production of human coagulation factor VIII. GenScript ProBio s...
Positive FDA meetings enable Neuren to proceed with INDs for three Phase 2 trials
Highlights: * Clear and constructive guidance received from pre-IND meetings with the FDA Office of Neuroscience for Phase 2 clinical trials of NNZ-2591 in Phelan-McDermid, Angelman andPitt Hopkins syndromes * Positive outcome is an important milestone that enables Neuren to proceed with IND...
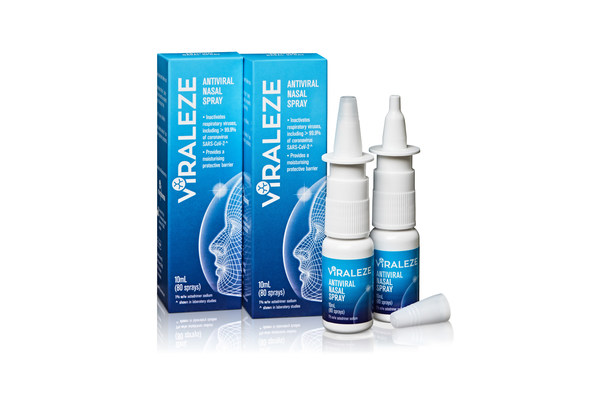
Scripps Research Institute testing shows Viraleze highly active against UK COVID strain
MELBOURNE, Australia, June 1, 2021 /PRNewswire/ -- Australia's Viraleze anti-COVID nasal spray is active against the highly infectious UK variant of SARS-CoV-2, the virus that causes COVID-19, reducing infectivity of the virus by >98%, it was announced today in a major development for the product...
Amgen and Kyowa Kirin to Jointly Develop and Commercialize KHK4083, a Phase 3-Ready, Potential First-In-Class Treatment for Atopic Dermatitis
Agreement Renews Successful Global Collaboration That led to Several Groundbreaking Therapies in Multiple Disease Areas KHK4083 Depletes OX40-Expressing T cells Associated with Atopic Dermatitis and Several Other Inflammatory Diseases Moderate-to-Severe Atopic Dermatitis Affects Nearly 30 Mill...
Innovent Biologics and AnHeart Therapeutics Jointly Announce Exclusive License Agreement for Taletrectinib in Greater China
SAN FRANCISCO and SUZHOU, China, June 1, 2021 /PRNewswire/ -- Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major disease...
Samsung Biologics to add mRNA vaccine drug substance manufacturing suite to expand portfolio of services
* Samsung Biologics, a global leading CDMO, will add mRNA vaccine DS production capability to its existing facility by the first half of 2022 * The addition will enable Samsung Biologics to provide end-to-end mRNA vaccine manufacturing services from bulk drug substance to aseptic fill/finish ...
Moleac Announces US FDA Approval of IND Application for Phase 1 Study of MLC1501 Programme in Post-Traumatic Brain Injury Recovery
SINGAPORE, May 31, 2021 /PRNewswire/ -- Moleac, a biopharmaceutical company based inSingapore, announced the approval by the U.S. Food and Drug Administration (FDA) of an investigational new drug (IND) application for MLC1501 in traumatic brain injury (TBI). Moleac is dedicated in finding, devel...
Hummingbird Bioscience Announces Publication of Abstract on Anti-VISTA Antibody HMBD-002 at the American Society of Clinical Oncology (ASCO) 2021 Annual Meeting
* HMBD-002 is the only IgG4 isotype anti-VISTA antibody currently in development * HMBD-002 is anticipated to enter a Phase 1 clinical trial in 2021 HOUSTON andSINGAPORE, May 31, 2021 /PRNewswire/ -- Hummingbird Bioscience, an innovative clinical-stage biotech company focused on developing pr...
Nexturn Bio Inc. acquired a 50% stake in RosVivo Therapeutics, Inc.
SEOUL, South Korea, May 30, 2021 /PRNewswire/ -- Nexturn Bio Inc., a subsidiary of Nexturn Bioscience Co., Ltd., announced onMay 26th that it had acquired a 50% stake in RosVivo Therapeutics Inc. ("Rosvivo"), an American new drug developer, for about5.5 million U.S. dollars. As a result, Nexturn...
Dr. Seungpil Yu will hold a retirement ceremony and conclude his 46-year term as a pharmaceutical executive.
SEOUL, South Korea, May 28, 2021 /PRNewswire/ -- Dr. Seungpil Yu is a living witness to the long history of Yuyu Pharma, which celebrated its 80th anniversary this year, having laid the foundation for Yuyu Pharma to continue to thrive well into its 100th year. While in the United States, Dr. Yu r...
Samsung Biologics Adds Four Global ISO Certifications For BCMS, Energy, Health & Safety, and Environmental Management
* Expanding upon its existing ISO22301 certification, Samsung Biologics is now fully certified with Business Continuity Management System for Security and Resilience across all business areas * Samsung Biologics adds ISO50001, ISO45001, ISO14001 for Energy Management, Occupational Health and ...
CStone Announces the First-in-Class Registrational Clinical Trial of Sugemalimab Met its Primary Endpoint in Stage III NSCLC and Plans to Submit a New Drug Application
* Sugemalimab becomes the world's first anti-PD-1/PD-L1 monoclonal antibody to successfully improve progression-free survival (PFS) in patients with stage III non-small-cell lung cancer (NSCLC) without disease progression after concurrent or sequential chemoradiotherapy * Sugemalimab is also t...
AffaMed Therapeutics Announces Approval for the Phase IIb Clinical Study of AM006 in China for the Treatment of Parkinson's Disease
SHANGHAI, May 27, 2021 /PRNewswire/ -- AffaMed Therapeutics ("AffaMed"), a global clinical stage biopharmaceutical company dedicated to addressing critical unmet medical needs in ophthalmic, neurological and psychiatric disorders, today announced thatChina's National Medical Products Administrati...
Viatris Signs Memorandum of Understanding with The Defeat NCD Partnership at the United Nations Institute for Training and Research (UNITAR)
Reveals initial findings of the Viatris sponsored report at a high-level panel discussion GENEVA and DUBAI, United Arab Emirates, May 28, 2021 /PRNewswire/ -- Viatris Inc. (NASDAQ: VTRS) and The Defeat-NCD Partnership (DNCD) at The United Nations Institute for Training and Research (UNITAR) toda...
Daewoong, first-in-class PRS-inhibitor for Idiopathic Pulmonary Fibrosis(IPF) shows promising result in early stage clinical test
* We confirmed the safety of 'DWN12088' and secured grounds for establishing treatment dosage * Confirming the potential as a treatment for IPF and planning to apply for Ph.2 within 2021 * 2nd ODD granted as treatment for systemic sclerosis from the US FDA SEOUL, South Korea, May 27, 2021...
ProBioGen and Minapharm Pharmaceuticals incorporate MiGenTra GmbH - A Healthcare Transforming Medicines Company
BERLIN and CAIRO, May 27, 2021 /PRNewswire/ -- ProBioGen and Minapharm Pharmaceuticals announce the incorporation of ProBioGen's subsidiary "MiGenTra" with headquarters inBerlin and access to a site in Cairo, which will house its principal manufacturing plant. The formation of MiGenTra accelerate...
Betagenon AB announces new CEO and new Chairman of the Board
STOCKHOLM, May 27, 2021 /PRNewswire/ -- Betagenon AB, a Sweden-based company focused on development of AMPK activator compounds, today announced that James Hall BM, BCh, D.Phil has joined this month as CEO. Dr. Hall brings extensive senior healthcare and life science experience, including 12 yea...
Foresee Pharmaceuticals Announces FDA Approval of CAMCEVI® for the Treatment of Advanced Prostate Cancer; Accord BioPharma to Head the U.S. Commercialization
TAIPEI, May 26, 2021 /PRNewswire/ -- Foresee Pharmaceuticals (6576.TWO), ("Foresee") announced today that the U.S. Food and Drug Administration (FDA) has approved the New Drug Application (NDA) for CAMCEVI® 42 mg, a ready-to-use 6-month subcutaneous depot formulation of leuprolide mesylate, as a ...
Week's Top Stories
Most Reposted
QDX Co-Founder Giuseppe Barca Awarded Prestigious Gordon Bell Prize, Pioneering Advancements in Drug Discovery Technology
[Picked up by 303 media titles]
2024-11-26 18:49ITAP 2024 sets the stage for a more connected advanced factory ecosystem with focus on AI, advanced robotics and sustainability
[Picked up by 296 media titles]
2024-11-28 17:24Southeast Asian Consumers Raise Online Security Concerns, New GSMA Survey Shows
[Picked up by 296 media titles]
2024-11-26 13:00TAILG's First Flagship Store in Indonesia Grandly Opens
[Picked up by 283 media titles]
2024-11-22 21:59MediSun Energy Raises $8.75M Seed Round with Vynn Capital to Drive MENA Expansion and Advance Osmotic Energy Innovation
[Picked up by 280 media titles]
2024-11-25 10:00