Pharmaceuticals
Lianhua Qingwen -- The Leading TCM In Global Anti-Pandemic Programs
SHIJIAZHUANG, China, March 11, 2021 /PRNewswire/ -- As an epitome of the Chinese civilization, traditional Chinese medicine or TCM, and western medicine, reinforce each other to protect and improve people's health. Since the outbreak of the COVID-19 pandemic,China has been strengthening the inte...
Ascentage Pharma to Present the Latest Results from Six Preclinical Studies at AACR Annual Meeting 2021
SUZHOU, China and ROCKVILLE, Md., March 11, 2021 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the latest preclinical...
OPTEL Group on Track for Record Year in Asia-Pacific Pharma Sales
OPTEL, a world leader in pharmaceutical track and trace for more than 30 years, expects the current fiscal year to be its best in terms of sales and project delivery to the pharmaceutical industry since the inauguration of its manufacturing plant inGoa, India, in 2016. GOA VELHA, India, March 1...
Impact Therapeutics Announced First-Patient-Dose of Wee1 Inhibitor IMP7068 in the U.S.
SHANGHAI, March 10, 2021 /PRNewswire/ -- Impact Therapeutics announced today that the first patient was recently dosed in the first-in-human Phase 1 clinical study of Wee1 Inhibitor IMP7068 inthe United States. This is a Phase 1, open-label, multi-center, dose escalation and expansion study to e...
Viva Biotech Successfully Held 2021 Partnership Summit -- Novel Drug 2021, the Persistence and Transformation of Start-up Founders
SHANGHAI, March 10, 2021 /PRNewswire/ -- March 2nd-6th, 2021, Viva Biotech successfully hosted the 2021 Partnership Summit. Over 300 attendees joined the Summit, including representatives from global investment institutions, R&D heads from pharmaceutical companies, and business development leader...
Sihuan Pharmaceutical (0460.HK): Be a friend of time, 2021 is the year of turning point
HONG KONG, March 10, 2021 /PRNewswire/ -- Sihuan Pharmaceutical Holdings Group Ltd. plays a pivotal role in the domestic medical industry. It is a leading company inChina's CCV prescription drugs. According to IMS data in 2018, Sihuan Pharmaceutical is well-known on the national hospital prescrip...
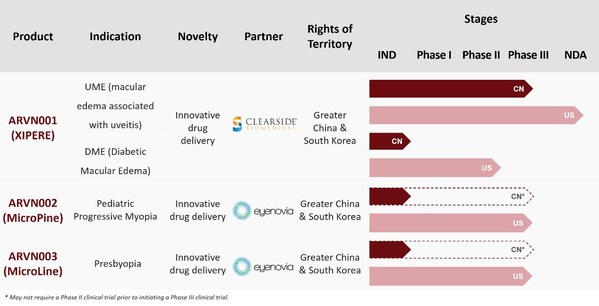
Arctic Vision Announces Completion of Over US$100 Million Series B Financing to Accelerate Portfolio Expansion, Clinical Development and Commercialization
SHANGHAI, March 9, 2021 /PRNewswire/ -- Arctic Vision, a China-based clinical-stage ophthalmology company focused on developing innovative therapies for pan-ocular diseases, today announced the completion of overUS$100 million Series B financing. The Series B round was led by Loyal Valley Capital...
Insilico's Chemistry42 AI system integrated into UCB's drug discovery programs
HONG KONG, March 9, 2021 /PRNewswire/ -- Insilico Medicine, an AI drug discovery company, announced that UCB will integrate Insilico'sChemistry42™ into UCB's internal drug discovery pipeline. UCB's early adoption of Insilico Medicine's proprietary technology will provide UCB's scientists with the...
Alphamab Received U.S. FDA IND Clearance to Initiate A Phase II Pivotal Clinical Trial of KN046 (KN046-205,ENREACH-Thymic) in the U.S.
SUZHOU, China, March 9, 2021 /PRNewswire/ -- Alphamab Oncology (stock code: 9966.HK) announced that U.S. Food and Drug Administration (FDA) has cleared the Company's Investigational New Drug application (IND) to initiate an open label, multi-center phase II pivotal clinical study (clinical trial ...
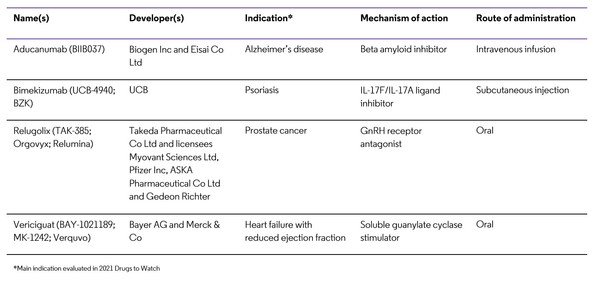
Clarivate Drugs to Watch Report Highlights Four Likely Blockbusters Among Drugs Launching in 2021
LONDON, March 9, 2021 /PRNewswire/ -- Clarivate Plc (NYSE:CLVT), a global leader in providing trusted information and insights to accelerate the pace of innovation, today announced the launch of its annual "Drugs to Watch" list, identifying drugs entering the market or launching key indications i...
Nippon Express (Malaysia) Obtains GDP Certification for Domestic Transport Services
TOKYO, March 9, 2021 /PRNewswire/ -- Nippon Express (Malaysia) Sdn, Bhd. (hereinafter, "NE Malaysia"), a local subsidiary of Nippon Express Co., Ltd. based inTokyo, has obtained Good Distribution Practice (GDP) certification effectiveThursday, January 14, 2021 for its Kuala Lumpur International A...
Yangtze River Pharmaceutical (Group) Co., Ltd. Won the 2021 EFQM Global Award
TAIZHOU, China, March 8, 2021 /PRNewswire/ -- At 19:20 on March 4, 2021 (Beijing time), the results of the 2021 EFQM Global Award were announced. Yangtze River Pharmaceutical (Group) Co., Ltd. fromChina was on the list, which represents that its quality management level has been aligned with that...
Winhealth Pharma and Merz Collaborate on Hepa-Merz® to benefit patients with liver disease in China
HANG ZHOU, China, March 8, 2021 /PRNewswire/ -- On Feb. 26th, 2021, Hongkong Winhealth Pharma Group Co., Ltd (hereinafter referred to as "Winhealth Pharma") signed a strategic cooperation agreement with Merz Pharmaceuticals GmbH (hereinafter referred to as "Merz"). The strategic cooperation betwe...
Entire industrial chain resources of advanced medical equipment are lining up at Medtec China 2021
SHANGHAI, March 8, 2021 /PRNewswire/ -- The advanced medical equipment industry chain includes various fields, such as materials, design, components, parts manufacturing, and complete machine manufacturing. Each field may lead to several subdivisions, where high scientific and technological insig...
FASN Inhibitor ASC40 Demonstrates Positive Phase 2 Topline Clinical Results from China Cohort of Patients with NASH
* Oral FASN inhibitor ASC40 shown to meaningfully reduce liver fat with a 50% responder rate * Consistent improvement in biomarkers of liver inflammation as observed in U.S. cohort SHANGHAI, China and SAN MATEO, California, March 9, 2021 /PRNewswire/ -- Gannex Pharma Co., Ltd., a wholly-owned...
AMD Telemed Applauded by Frost & Sullivan for Its Modular Engage N' Solution that Supports Patient Needs by Acuity Level
AMD's unified virtual care framework connects interoperable electronic health records, medical devices, and various engagement models to offer exceptional value to clients SANTA CLARA, Calif., March 9, 2021 /PRNewswire/ -- Based on its recent analysis of the North American virtual care market,Fr...
Wugen Announces Exclusive Partnership Agreement With Alpha Biopharma in Asia For Cell Therapies to Treat Cancer
ST. LOUIS, March 8, 2021 /PRNewswire/ -- Wugen Inc., a clinical-stage biotechnology company developing novel universal natural killer (NK) and T-cell therapies for the treatment of cancer, today announced that it has entered into an exclusive license and collaboration agreement withShanghai-based...
CMAB Biopharma Congratulates Partner Junshi Biosciences on NMPA Acceptance of Application for a Clinical Trial of its PD-1/TGF-β Bifunctional Fusion Protein
SUZHOU, China, March 7, 2021 /PRNewswire/ -- Recently, CMAB Biopharma (Suzhou) Inc's ("CMAB") partner Shanghai Junshi Biosciences Co., Ltd. ("Junshi Biosciences") (HK: 1877; SH: 688180), announced that a clinical trial application for its PD-1/TGF-β bifunctional fusion protein JS201 injection (J...
Adlai Nortye Announces Formation of its New Scientific Advisory Board
HANGZHOU, China, March 7, 2021 /PRNewswire/ -- Adlai Nortye, a global clinical-stage biopharmaceutical company, today announced the formation of its new Scientific Advisory Board (SAB) comprised of five internationally renowned experts. The SAB includesRonald M. Evans, PhD (Member of the US Natio...
Patients Win When Support Is Personalized: New Study From Atlantis Healthcare Shows Treatment Persistence and Adherence Improved by 38%
SYDNEY, March 8, 2021 /PRNewswire/ -- Leading patient outcomes journal Patient
Preference and Adherence
Week's Top Stories
Most Reposted
Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 328 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Amadeus acquires SkyLink to accelerate the deployment of AI in travel
[Picked up by 310 media titles]
2026-02-26 19:57HBX Group and Traveloka expand strategic partnership to deepen APAC supply and accelerate global distribution
[Picked up by 309 media titles]
2026-02-26 09:30Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13