Health
Tenth Record-Breaking Year in a Row for ZEISS
STUTTGART, Germany, Dec. 11, 2019 /PRNewswire/ -- The ZEISS Group continued its growth trajectory, with revenue and earnings reaching new heights in fiscal year 2018/19, which ended30 September 2019. Revenue grew by 11 percent, reaching 6.428 billion euros (previous year: 5.817 billion euros). Adj...
GemVax announced phase II results of GV1001 for Alzheimer's disease treatment at CTAD 2019
SEOUL, South Korea, Dec. 11, 2019 /PRNewswire/ -- The 12th Clinical Trials on Alzheimer's Disease (CTAD) 2019 was held inSan Diego, USA, from December 4-7. At the CTAD, the results of various Alzheimer's disease clinical trials were shared, and it provided a glimpse into the possibility of treati...
Xinhua Silk Road: E. China Nanjing Jiangbei New Area aims to develop life and health industry
BEIJING, Dec. 11, 2019 /PRNewswire/ -- Nanjing Jiangbei New Area, a state-level new area in East China'sJiangsu Province, is committed to building a 100 billion-yuan level life and health industrial cluster and becoming a gene city inChina. The news was announced by Luo Qun, a member of the Stan...
West China Hospital to build new hospital in Chengdu Tianfu New Area
CHENGDU, China, Dec. 11, 2019 /PRNewswire/ -- West China Hospital of Sichuan University, a prestigious medical center located inChengdu, Sichuan province, signed an agreement on Dec. 5 with the Chengdu Management Committee of Tianfu New Area, China's 11th national-level development area, to build...
CStone announces enrollment target reached in the global Phase III VOYAGER trial of avapritinib in Chinese patients with third-line GIST
SUZHOU, China, Dec. 11, 2019 /PRNewswire/ -- CStone Pharmaceuticals ("CStone" or the "Company", HKEX: 2616) today announced that the on-going, global Phase III VOYAGER clinical trial of avapritinib, an investigational drug discovered by CStone's partner, Blueprint Medicines, has completed target ...
AGC Biologics Expands Plasmid DNA Offering
SEATTLE, Dec. 11, 2019 /PRNewswire/ -- AGC Biologics, a global Contract Development and Manufacturing Organization (CDMO) for Biopharmaceuticals, is significantly expanding its CDMO services with plasmid DNA (pDNA) offerings from itsHeidelberg site in Germany. With the demand for pDNA growing rap...
Kazia Initiates Preparatory Activities to Bring GDC-0084 into GBM AGILE, an International Phase II / III Study in Glioblastoma
SYDNEY, Dec. 11, 2019 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to announce that it's lead program, GDC-0084, has been selected to join GBM AGILE, an international, academic-led, multi-drug adaptive phase ...
Ascentage Pharma Releases Updated Data of its Novel BCR-ABL Inhibitor, HQP1351, in an Oral Presentation Nominated for "Best of ASH"
SUZHOU, China and ROCKVILLE, Md., Dec. 11, 2019 /PRNewswire/ -- Ascentage Pharma (6855.HK), a globally-focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that the updated results ...
Vision Impact Institute: New Reports on State of Vision Will Renew Global Focus on the Societal Impact of Poor Vision
First WHO World Health Report on Vision and Essilor Report on Eliminating Poor Vision underscore need to include vision health in global public health agenda DALLAS, Dec. 11, 2019 /PRNewswire/ -- The Vision Impact Institute welcomes the release of two new complementary and aligned reports on t...
Conversa Honored by Frost & Sullivan for its Clinically Intelligent Connectivity Platform that Improves Care, Enhances Patient and Provider Experience
Conversa has built a game-changing, real-time, "patient signals" model SANTA CLARA, Calif., Dec. 10, 2019 /PRNewswire/ -- Based on its recent analysis of the North American patient care management market, Frost & Sullivan recognizesConversa Health with the 2019 North American Visionary Innovation...
PointClickCare Integrated Care Coordination Platform Applauded by Frost & Sullivan for Bridging the Communication Gap between Acute and Post-acute Healthcare Providers
Harmony by PointClickCare Enables Coordinated and Collaborative Care Delivery on a Secure and Integrated platform SANTA CLARA, California, Dec. 10, 2019 /PRNewswire/ -- Based on its recent analysis of the North American long-term and post-acute care (LTPAC) IT management market, Frost & Sullivan...
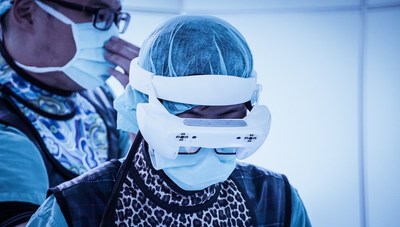
Breakthrough in Medical Electronics - A Novel Mixed & Augmented Reality Smart Glasses Surgical Navigation Solution
TAICHUNG, Dec. 10, 2019 /PRNewswire/ -- The mixed reality technology for surgery usually shown in the movies has now become a reality with the latest technological offering from theTaiwan-based company – Taiwan Main Orthopaedics Biotechnology and the branding name of medical devices is SURGLAS...
Results of Fully-human BCMA CAR-T for the Treatment of Relapsed/Refractory Multiple Myeloma Co-developed by Innovent and IASO BIO Presented at the 2019 ASH Annual Meeting
SUZHOU, China, Dec. 10, 2019 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops and commercializes high quality medicines for the treatment of oncology, autoimmune, metabolic and other major diseases, today announced that th...
CStone's anti-PD-L1 antibody demonstrates promising antitumor activity with a complete response rate of 33.3% and a good safety profile in patients with relapsed or refractory extranodal natural killer/T-cell lymphoma
SUZHOU, China, Dec. 10, 2019 /PRNewswire/ -- CStone Pharmaceuticals ("CStone" or the "Company", HKEX: 2616) updated results from the CS1001-201 trial in a poster presentation at the 2019 American Society of Hematology (ASH) Annual Meeting. CS1001-201 trial is a single-arm, multicenter Phase II c...
Farrer Park Hospital Implements Non-Invasive Treatment for Prostate Cancer Patients in Singapore
SINGAPORE, Dec. 10, 2019 /PRNewswire/ -- Farrer Park Hospital is pleased to announce that it is the first inSouth East Asia to have implemented the Focal One® High-Intensity Frequency Ultrasound (HIFU) Robotic Technology, a non-invasive prostate cancer treatment, for patients with early-stage pro...
Townsville Hospital Treats First Australian Patient With Elekta Unity MR-Linac, a Transformative Approach to Precision Radiation Therapy
Ability to adapt daily treatment based on real-time MR imaging enables new approach to hard-to-treat cancers TOWNSVILLE, Australia, Dec. 10, 2019 /PRNewswire/ -- Elekta (EKTA-B.ST) today announced that Townsville Hospital inQueensland has treated the first patient in Australia using Elekta Unity ...
TechMah Medical & LimaCorporate Receive First 510k Approval for Smart SPACE Digital Technology
UDINE, Italy, Dec. 10, 2019 /PRNewswire/ -- LimaCorporate is pleased to announce the FDA510K approval for Smart SPACE Shoulder 3D Planner & 3D Positioner. Smart SPACE is an innovative digital platform developed by TechMah Medical thanks to collaboration with LimaCorporate, who entered into a mil...
Gracell Announces Progressive Outcomes from Multiple Human Clinical Trials to Investigate FasTCAR and Dual CAR Cell Platform Technologies
SUZHOU, China and SHANGHAI, Dec. 9, 2019 /PRNewswire/ -- Gracell Biotechnologies Co., Ltd ("Gracell"), a clinical-stage immune cell therapy company, today announced the progressive clinical outcomes for leading product candidates FasTCAR-19, Dual CAR-19-22, and Dual CAR-BCMA-19 at the American...
Publication of First In-Human Data Reveals Safety & Efficacy of Tigilanol Tiglate in Solid Tumours
* First in-human Clinical Phase I study of intratumoural tigilanol tiglate (EBC-46) has been published in The Lancet's EBioMedicine journal; * An efficacious dose was achieved and a Maximum Tolerated Dose (MTD) was not reached, indicating tigilanol tiglate tolerability in humans; * Promising...
BJ Bioscience Announces First Patient Dosed in FIH Trial of BJ-001 in Patients with Locally Advanced/Metastatic Solid Tumors
HANGZHOU, China, Dec. 9, 2019 /PRNewswire/ -- BJ Bioscience Inc. (the
'company') today announced that the first patient was successfully dosed on
December 4, 2019 in the first-in-human (FIH) trial of the company's BJ-001
program at NEXT ONCOLOGY inSan Antonio, Texas.
Week's Top Stories
Most Reposted
Rocket Travel by Agoda Shares Revealing New Report and Showcases Solution to Transform Hotel Distribution
[Picked up by 313 media titles]
2024-11-21 10:30Rockwell Automation and Microsoft Deliver on a Shared Vision to Accelerate Industrial Transformation
[Picked up by 308 media titles]
2024-11-20 13:29Durabook and Parent Company, Twinhead International Corp., Celebrate 40 Years of Innovation in Computing Solutions
[Picked up by 301 media titles]
2024-11-20 16:30Travel loyalty programs to focus on offering personalized and flexible customer experiences in 2025
[Picked up by 298 media titles]
2024-11-19 10:42Philips and Edith Cowan University Australia Collaborate to Equip the Next Generation of Healthcare Professionals to leverage new technologies
[Picked up by 284 media titles]
2024-11-20 09:00