Biotechnology
Kira Pharmaceuticals Presents Complete Data from Phase 1 SYNERGY-1 Trial of KP104 at American Society for Nephrology Kidney Week 2022
Biomarker data demonstrates proof-of-concept for KP104's dual-targeting mechanism via dose-dependent inhibition of alternative and terminal pathways and supports intravenous and subcutaneous administration of KP104 in phase 2 studies CAMBRIDGE, Mass. and SUZHOU, JIANGSU, China, Nov. 3, 2022 /PRN...
DOUBLE HONORS FOR AMGEN WITH GREAT PLACE TO WORK™ CERTIFICATIONS FOR BOTH ITS SINGAPORE ENTITIES IN 2022
SINGAPORE, Nov. 3, 2022 /PRNewswire/ -- Amgen announced both its entities in Singapore - Amgen Singapore Manufacturing (ASM) and its commercial affiliate Amgen Biotechnology Singapore (ABS), have been awarded Great Place to Work® certifications in the 'medium and large' workplace category for 202...
NeuroXess Shares Latest Breakthoughs in Life Sciences at Sequoia Talk
SHANGHAI, Nov. 3, 2022 /PRNewswire/ -- Peng Lei, CEO of NeuroXess, a Sequoia firm, was invited to participate in Sequoia China's Sequoia Talk Series on "The Compound Interest of Innovation" at which he shared his knowledge of and experience with the latest technological innovations in the field o...
Turn Biotechnologies Names 25-year Biotech Industry Veteran Richard Peterson Chief Financial Officer
Seasoned Executive has Successful Track Record Working with Global Investors to Raise Billions of Dollars MOUNTAIN VIEW, Calif., Nov. 2, 2022 /PRNewswire/ -- Turn Biotechnologies, a cell rejuvenation company developing novel mRNA medicines to cure untreatable, age-related conditions, today annou...
Antengene Announces IND Approval for the Phase I STAMINA-001 Study to Evaluate ATG-037 (CD73 Inhibitor) for the Treatment of Locally Advanced or Metastatic Solid Tumors in China
- ATG-037, an inhouse asset developed by Antengene and with global rights, has been approved to enter clinical studies inAustralia and China, thus becoming the firstoral small molecule CD73 inhibitor entering the clinical-stage in China and the wider Asia Pacific region. ATG-037 IND in Austr...
Dr Amit Kakar joins MedGenome Board of Directors
* The company has also added Navjeewan Khosla from Novo Holdings as Board Observer. * The company had announced a $50 million Series D investment led by Novo Holdings inAugust 2022. BANGALORE, India, Nov. 2, 2022 /PRNewswire/ -- MedGenome is a leading provider of genomic solutions for populat...
Ascletis Announces U.S. IND Filing of Oral 3CLpro Inhibitor ASC11 for COVID-19
-- ASC11 is an in-house discovered oral small molecule drug candidate, targeting 3-chymotrypsin like protease (3CLpro) -- In antiviral cellular assays with infectious SARS-CoV-2, ASC11 demonstrated higher potency against SARS-CoV-2 than other 3CLpro inhibitors including Nirmatrelvir, S-217622, P...
SK pharmteco appoints Joerg Ahlgrimm as CEO to spearhead the next phase of company growth and innovation
- Joerg Ahlgrimm brings 25 years of expertise in pharmaceuticals and innovative cell and gene therapies - SK pharmteco expands pharmaceutical drug production and advances cell and gene therapy production - Annual sales expected to exceed KRW 1 trillion in 2022 FRANKFURT, Germany, Nov. 2, 2022 /PR...
Henlius' Novel Anti-PD-1 mAb HANSIZHUANG (Serplulimab) Receives NMPA Approval for the Treatment of sqNSCLC
SHANGHAI, Nov. 1, 2022 /PRNewswire/ -- Shanghai Henlius Biotech, Inc. (2696. HK) announced that its first self-developed innovative anti-PD-1 monoclonal antibody (mAb) HANSIZHUANG (generic name: serplulimab injection), in combination with carboplatin and albumin-bound paclitaxel for the first-lin...
Immuno Cure appoints new CFO
Strengthening of executive management team to propel growth strategy HONG KONG, Nov. 1, 2022 /PRNewswire/ -- Immuno Cure BioTech, a clinical stage biotechnology group based in the Hong Kong Science Park specialising in DNA vaccines and immunotherapies, announced today the appointment of Mr M...
Hummingbird Bioscience to Attend the Credit Suisse 31st Annual Healthcare Conference
SAN FRANCISCO, HOUSTON and SINGAPORE, Nov. 1, 2022 /PRNewswire/ -- Hummingbird Bioscience, a data-driven precision biotherapeutics company discovering and developing transformative biologic medicines for hard-to-treat diseases, today announced that Chief Financial OfficerJosh House and Chief Deve...
Karyopharm and Menarini Group Announce Orphan Medicinal Product Designation from the European Commission for Selinexor for the Treatment of Myelofibrosis
NEWTON, Mass. and FLORENCE, Italy, Nov. 1, 2022 /PRNewswire/ -- Karyopharm Therapeutics Inc. (NASDAQ: KPTI), a commercial-stage pharmaceutical company pioneering novel cancer therapies, and the Menarini Group ("Menarini"), a privately-held, leading international pharmaceutical company, today anno...
Citeline and Norstella Complete Merger to Form a $5 Billion Global Pharmaceutical Technology Company
With more than 1,600 employees across the globe, the new organization is now one of the world's largest pharma intelligence solutions providers on the market YARDLEY, Pa., Nov. 1, 2022 /PRNewswire/ -- Norstella, a global leader with end-to-end solutions that smooth access to life-saving therapies...
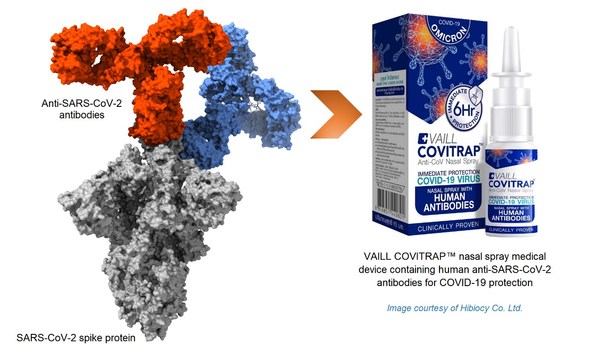
GenScript Supported Nasal Spray Development for COVID-19 Protection with GMP-grade Antibodies and Subsequently Planned for Long-Term Collaboration with Biogenexis
SINGAPORE, Nov. 1, 2022 /PRNewswire/ -- GenScript Biotech Corporation ("GenScript", Stock Code: 1548.HK), a leading global biotechnology company supportedChulalongkorn University, a research-intensive university in Thailand in developing human anti-SARS-CoV-2 antibodies with antibody production a...
Antengene Receives U.S. FDA Clearance of IND Application for Phase I Trial of Small Molecule ERK1/2 Inhibitor ATG-017 in Patients with Advanced Solid Tumors
* ATG-017 is a small molecule ERK1/2 inhibitor and Antengene has obtained exclusive global rights to develop, commercialize and manufacture ATG-017. * IND clearance enables Antengene to initiate the combination portion of the Phase I "ERASER" clinical trial inthe United States (U.S.) to evalua...
EpiVax Joins Intravacc, CEPI on Project to Develop Universal Betacoronavirus Vaccine
PROVIDENCE, R.I., Oct. 31, 2022 /PRNewswire/ -- EpiVax, Inc. ("EpiVax") is pleased to announce participation in a new project with Intravacc, funded by CEPI (Coalition for Epidemic Preparedness Innovations), to develop universal vaccines for betacoronaviruses, including SARS-CoV-2. EpiVax will be...
BRONCUS'S DISPOSABLE NEBULIZING MICRO-CATHETER FOR ENDOSCOPE WAS APPROVED FOR MARKETING IN CHINA AND ITS APPLICATIONS IN DRUG-DEVICE COMBINATION WILL BE BROADENED
HANGZHOU, China, Oct. 31, 2022 /PRNewswire/ -- Broncus is pleased to announce that onOctober 28, 2022, the Company's innovative product in the field of drug-device combination, "Mist FountainTM" (the "Product" or "Nebulizing Micro-Catheter"), a disposable nebulizing micro-catheter for endoscope, ...
Hinova Disclosed the Preclinical Results of HP518 (Oral AR PROTAC) at the 5th Annual TPD Summit
BOSTON, Oct. 29, 2022 /PRNewswire/ -- Hinova Pharmaceuticals Inc. (STAR: 688302), a clinical-stage biopharmaceutical company focused on developing novel therapeutics for cancers and metabolic diseases through targeted protein degradation technologies, today presented their preclinical results of ...
Pharming Announces European Medicines Agency (EMA) Validates its Marketing Authorisation Application under Accelerated Assessment for leniolisib
Marketing authorisation in the European Economic Area anticipated in H1 2023 LEIDEN, Netherlands, Oct. 28, 2022 /PRNewswire/ -- Pharming Group N.V. ("Pharming" or "the Company") (EURONEXT Amsterdam: PHARM / Nasdaq: PHAR) announces today that its Marketing Authorisation Application (MAA) for leni...
Acceptance Of The Application For Clinical Trial Of Recbio Novel Adjuvanted Recombinant Quadrivalent HPV Vaccine
TAIZHOU, China, Oct. 28, 2022 /PRNewswire/ -- Jiangsu Recbio Technology Co., Ltd. (the "Company", together with its subsidiaries, the "Group") is pleased to announce that, the Group has recently received a notice of acceptance from the National Medical Products Administration to accept the appli...
Week's Top Stories
Most Reposted
Earth Day 2024: Angel Yeast Continues to Tackle Plastic Pollution Challenges With Bio-based Material Solutions
[Picked up by 290 media titles]
2024-04-22 16:00Trina Solar and PetroGreen Partner to Accelerate Philippine Solar Adoption with 117MW Supply Agreement
[Picked up by 289 media titles]
2024-04-22 06:00With unique features, Hainan TV opened "The World's Specialty" pavilion at the Expo!
[Picked up by 287 media titles]
2024-04-18 21:26China's Yiwu Establishes Welcoming Committee to Attract International Buyers
[Picked up by 286 media titles]
2024-04-19 13:25Animated Life in Yunnan
[Picked up by 281 media titles]
2024-04-18 14:00