Biotechnology
RedHill Biopharma Initiates Phase 3 Study of RHB-204 for First-Line Treatment of NTM Disease
RHB-204 is being evaluated as a first-line, stand-alone, oral treatment for pulmonary nontuberculous mycobacteria (NTM) disease - a rare condition with no FDA-approved first-line therapy The Phase 3 study is expected to recruit up to 125 patients across approximately 40 U.S. clinical sites RHB-...
WuXi Biologics Launched Biologics Integrated Innovation Center in Hangzhou
HANGZHOU, China, Nov. 20, 2020 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, today announced its biologics integrated innovation center has been in operation inHangzhou, Zhejiang province, China. From process deve...
RedHill Biopharma's Second COVID-19 Candidate, RHB-107, Cleared by FDA for Phase 2/3 Study in Symptomatic COVID-19 Disease
FDA clears IND application for Phase 2/3 study with RedHill's second novel COVID-19 candidate, RHB-107 (upamostat), an orally administered novel serine protease inhibitor, with demonstrated antiviral and potential tissue-protective effects The Phase 2/3 study is designed to evaluate outpatient-b...
Covis Group Announces Data for Ciraparantag Published in Blood, the Journal of the American Society of Hematology
Summarizes ciraparantag's expected mechanism of action, pharmacokinetic, and pharmacodynamic characteristics and its proof of concept as an anticoagulant reversal agent LUXEMBOURG and ZUG, Switzerland, Nov. 20, 2020 /PRNewswire/ -- Covis Group S.à r.l. ("Covis") today announced that data demonst...
RedHill Announces Unanimous DSMB Recommendation to Continue Phase 2/3 COVID-19 Study with Opaganib
Pre-scheduled independent Data and Safety Monitoring Board (DSMB) unanimously recommends continuation of the global Phase 2/3 study of orally administered opaganib in severe COVID-19 pneumonia Enrollment in the 270-patient global Phase 2/3 COVID-19 study with opaganib is more than 50% complete ...
With Elekta Studio, Elekta brings complete image-guided brachytherapy workflow to a single room
VEENENDAAL, The Netherlands, Nov. 19, 2020 /PRNewswire/ -- Elekta (EKTA-B.ST) today introduced Elekta Studio with its launch of the ImagingRing, an advanced interventional CT system that revolves around the patient and enables clinicians to conduct the entire brachytherapy workflow - including ap...
Mibelle Biochemistry's Ability to Introduce Breakthrough Active Ingredients for the Personal Care Market Lauded by Frost & Sullivan
The company's active ingredient product lines meet consumer expectations of quality, performance, and sustainability LONDON, Nov. 18, 2020 /PRNewswire/ -- Based on its recent analysis of the global personal care active ingredients market, Frost & Sullivan recognizes Mibelle AG Biochemistry with t...
Deep Longevity Launches The First Longevity Medicine Course For Physicians
HONG KONG, Nov. 18, 2020 /PRNewswire/ -- Regent Pacific Group Limited ("Regent Pacific" or the "Company" and together with its subsidiaries, the "Group"; SEHK:0575.HK)'s Deep Longevity, Inc, a company subject to a conditional acquisition by the Group which is a pioneer in deep biomarkers of aging...
Global Cord Blood Corporation to Report Second Quarter and First Half Fiscal 2021 Financial Results
HONG KONG, Nov. 18, 2020 /PRNewswire/ -- Global Cord Blood Corporation (NYSE: CO) (the "Company"),China's leading provider of cord blood collection, laboratory testing, hematopoietic stem cell processing and stem cell storage services, today announced that it plans to release unaudited financia...
HistoIndex's AI-Based Platform Demonstrates Zonal Quantification and Histological Characterization of Fibrosis Improvements in Patients With Nonalcoholic Steatohepatitis (NASH)
SINGAPORE, Nov. 18, 2020 /PRNewswire/ -- HistoIndex
Chinese biotech company releases rapid COVID-19 antigen test with easy-to-use nasal swab
BINHAI, China, Nov. 18, 2020 /PRNewswire/ -- JOYSBIO Biotechnology Co., Ltd. announced today that they have introduced a new rapid COVID-19 antigen test kit available to medical facilities around the world as well as direct-to-consumer for at-home testing depending on local legislation. The kit ...
Kazia Presents Further Paxalisib Data At Sno, Confirming Earlier Positive Safety And Efficacy Signals In Glioblastoma
SYDNEY, Nov. 18, 2020 /PRNewswire/ -- Kazia Therapeutics Limited (ASX: KZA; NASDAQ: KZIA), an Australian oncology-focused biotechnology company, is pleased to share a summary of new paxalisib data presented at the Society for Neuro-Oncology (SNO) Annual Meeting, which is being held virtually from...
Foxx Life Sciences Announces Opening of New Asia Headquarters in India
SALEM, New Hampshire, Nov. 17, 2020 /PRNewswire/ -- Foxx Life Sciences announced on Tuesday that it will open the new internationalAsia headquarters in Medchal,Hyderabad, India. The latest expansion comes with strong growth and performance inAsia and the continued expansion of Biopharmaceutical ...
Visiopharm's AI-Powered Image Analysis Software Selected to Analyze the Largest Collection of Human Pathology Specimens in the World
DENVER and HOERSHOLM, Denmark, Nov. 17, 2020 /PRNewswire/ -- Visiopharm, the leader in artificial intelligence-driven image analysis, tissue mining, and precision pathology, has been selected by the Joint Pathology Center (JPC), the premier pathology reference center forthe United States federal ...
INOVIO Announces Initiation of Phase 2 Segment of its Phase 2/3 Clinical Trial for its COVID-19 DNA Vaccine Candidate, INO-4800; Trial Will Be Funded by the U.S. Department of Defense
PLYMOUTH MEETING, Pa., Nov. 16, 2020 /PRNewswire/ -- INOVIO (NASDAQ:INO), a biotechnology company focused on bringing to market precisely designed DNA medicines to treat and protect people from infectious diseases and cancer, today announced that it has received clearance from the U.S. Food & Dru...
Cytiva supports Clover Biopharmaceuticals to scale up the output of its vaccine candidate
* Clover aims to double its commercial manufacturing capacity in preparation for global Phase 2/3 trials * Cytiva provides FlexFactory™ bioprocessing upstream and downstream solutions for Clover to accelerate GMP facility development and shorten time to market SHANGHAI, Nov. 16, 2020 /PRNewsw...
Innovent Announces the Results of the Phase I Clinical Trial of IBI302, a First-in-class Ophthalmic Recombinant Human Anti-VEGF and Anti-Complement Bi-specific Fusion Protein for Neovascular Age-Related Macular Degeneration at 2020 American Academy of Ophthalmology
SAN FRANCISCO and SUZHOU, China, Nov. 16, 2020 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, metabolic, autoimmune and other major di...
The third CIIE concludes, $72.6 billion in intended deals for the coming year inked
SHANGHAI, Nov. 14, 2020 /PRNewswire/ -- Despite the global market uncertainties caused by the COVID-19 pandemic, the confidence and interest that foreign companies have in the Chinese market appear unchanged, with the value of tentative deals reached for one-year purchases of goods and services a...
Positive Results from RhoVac's Clinical Phase I/II Study published in Journal of ImmunoTherapy of Cancer
STOCKHOLM, Nov. 13, 2020 /PRNewswire/ -- RhoVac announces today, November 13, 2020, that the results of its clinical phase I/II study have been published in the Journal for ImmunoTherapy of Cancer (JITC). The scientifically reviewed article reports that the treatment with RhoVac's drug candidate ...
Molecular Stethoscope Announces Completion of its NAFLD/NASH Study and Podium Presentation of Results by Dr. Naga Chalasani at the AASLD Liver Meeting 2020
Study results demonstrate the utility of the company's Next-Generation cf-mRNA Liquid Biopsy-Artificial Intelligence Technology Platform for the characterization of NAFLD/NASH disease PALO ALTO, Calif., Nov. 13, 2020 /PRNewswire/ -- Molecular Stethoscope, Inc., a precision medicine biotechnology...
Week's Top Stories
Most Reposted
LONG AN INTERNATIONAL PORT JOINS 12TH PORTECH ASIA SUMMIT 2025 IN MALAYSIA
[Picked up by 327 media titles]
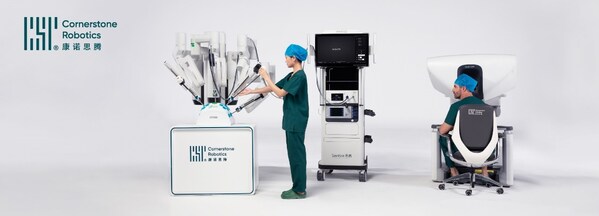
2025-01-18 03:30Cornerstone Robotics Raises over US$70 million Funding to Forge Accessibility in Robotic Surgery
[Picked up by 323 media titles]
2025-01-13 09:05Hong Kong Airlines Takes Off to Australia's Gold Coast Bringing Popular Travel Option for the Chinese New Year
[Picked up by 298 media titles]
2025-01-18 17:00RuggON Launches VIKING II: Transforming Fleet Management with Unmatched Technology and Flexibility
[Picked up by 295 media titles]
2025-01-14 21:00URBAN REVIVO Celebrates Lunar New Year with Unique Collaboration with Artist Mercedes Bellido
[Picked up by 294 media titles]
2025-01-13 10:44