Biotechnology
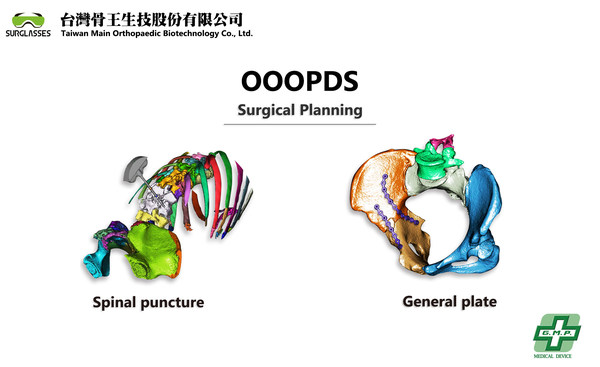
OOOPDS - a surgical planning software received TFDA Class-II medical device certification developed by Taiwan Main Orthopaedic Biotechnology Co., Ltd.
TAICHUNG, Taiwan, April 12, 2021 /PRNewswire/ -- Taiwan Main Orthopaedic Biotechnology announced today (12) that their OOOPDS 3D medical image reconstruction and surgical planning medical software has received TFDA Class-II certification. OOOPDS is the most comprehensive 3D surgical planning sof...
Airway Therapeutics Announces FDA Acceptance of IND for AT-100's Second Indication in Severe COVID-19 Patients
Initiating Phase 1b clinical trial with initial results expected 3Q2021 Potential for novel therapeutic AT-100 to inhibit viral replication, promote viral elimination and reduction of lung injury, inflammation, and secondary infections in severe COVID-19 patients CINCINNATI, April 12, 2021 /PRNe...
GenScript, Parkway Laboratories and Diagnostics Development (DxD) Hub collaborate to provide cPass(TM) SARS-CoV-2 Neutralizing Antibody Testing Service in Singapore
SINGAPORE, April 12, 2021 /PRNewswire/ -- GenScript Biotech Corporation ("GenScript", Stock Code: 1548.HK), Parkway Laboratories and the Diagnostics Development (DxD) Hub announced their collaboration to provide the cPass™ SARS-CoV-2 neutralizing antibody test inSingapore through Parkway's pane...
CAVAC Takes the Lead in Worldwide Anti-rabies Vaccination
SEOUL, South Korea, April 12, 2021 /PRNewswire/ -- The Ministry of SMEs and Startups (MSS) and BSR Korea are encouraging and supporting decent and respectful domestic SMEs to enter the global procurement-as-a-service market, with the project Marketing Support for Companies Specialized in Bio-func...
Antengene Announces First Patient Dosed in Phase II Trial of ATG-008 (Onatasertib) in Patients with Advanced Solid Tumors with Specific Genetic Alterations
SHANGHAI and HONG KONG, April 12, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in-class therapeutics in hematology and onco...
Hummingbird Bioscience Presents Pre-Clinical Data on its Next-Generation BCMA-TACI Dual-Specific T Cell Engager at 2021 AACR Annual Meeting
SINGAPORE, April 10, 2021 /PRNewswire/ -- Hummingbird Bioscience, an innovative clinical-stage biotech company focused on developing precision therapies against hard-to-drug targets to improve treatment outcomes, today announced that the company will be presentingpre-clinical data on its BCMA-TAC...
RedHill Biopharma's Phase 2/3 COVID-19 Study of Opaganib Passes Fourth DSMB Review with Unanimous Recommendation to Continue
Independent Data Safety Monitoring Board unanimously recommends continuation of the global Phase 2/3 study of orally-administered opaganib in severe COVID-19 pneumonia based on review of unblinded safety data from the first 255 treated patients The 464-patient global Phase 2/3 COVID-19 study is ...
Remiges Ventures Closes Second Fund With $95 Million; Launches RDiscovery Incubator
Remiges Ventures, a US-based cross-border venture capital firm with offices in Seattle, WA and Tokyo, Japan, announced today the final closing of its second fund, Remiges BioPharma Fund II raising a total of$95 million, and the launch of RDiscovery, a new life sciences incubator to nurture and ad...
Shanghai Genechem Co., Ltd. (Genechem) Announces appointment of Dr. Eric Rowinsky and Dr. Manuel Hidalgo to Scientific Advisory board, and abstract presentation at AACR 2021 on Claudin 6 Ab and K-Ras TCR-T
SHANGHAI, April 8, 2021 /PRNewswire/ -- Shanghai Genechem Co., Ltd. (Genechem), a discovery company dedicated to novel target discovery and development of novel therapeutics, today announced the appointment of Dr.Eric Rowinsky, President and Executive Chairman of the Board of Rgenix, Inc., and Dr...
Antibiotic Alternatives Stimulate New Product Development in Global Animal Feed Ingredient Market
Amino acids dominate the global industry and are expected to generate revenues of$11.5 billion by 2026, says Frost & Sullivan SANTA CLARA, Calif., April 8, 2021 /PRNewswire/ -- Frost & Sullivan's recent analysis finds that the rising demand forhigh-quality meat products across the globe, especia...
Inmagene R&D Team expands with two new senior members joining the Company
SAN DIEGO and SHANGHAI and HANGZHOU, China, April 7, 2021 /PRNewswire/ -- Inmagene Biopharmaceuticals ("Inmagene") today announced that Dr.Anbo Xiang and Dr.Qian Xu joined the company. As CMO (China) and Senior VP, Operations, Dr. Xiang will be responsible for clinical development, operations, an...
WuXi Biologics Successfully Completed Pre-License Inspection and Routine GMP Inspection by U.S. FDA
WUXI, China, April 7, 2021 /PRNewswire/ -- WuXi Biologics ("WuXi Bio") (2269.HK), a global company with leading open-access biologics technology platforms, today announced that it has successfully completed both a Pre-License Inspection (PLI) and a routine GMP inspection by the U.S. FDA (FDA). T...
Antengene Announces NMPA Approval of IND Application for ATG-019 in Patients with Advanced Solid Tumors or Non-Hodgkin's Lymphoma
SHANGHAI and HONG KONG, April 6, 2021 /PRNewswire/ -- Antengene Corporation Limited ("Antengene", SEHK: 6996.HK), a leading innovative biopharmaceutical company dedicated to discovering, developing and commercializing global first-in-class and/or best-in class therapeutics in hematology and oncol...
Novavax Initiates COVID-19 Vaccine Clinical Trial Crossover
* Crossover allows participants to continue in trials and remain blinded * Ensures that all trial participants receive active vaccine * South Africa and UK crossover arms initiated; US/Mexico PREVENT-19 crossover planned GAITHERSBURG, Md., April 6, 2021 /PRNewswire/ -- Novavax, Inc. (Nasdaq:...
Thailand BOI Okays Biotech Projects Worth 2.4 Bln Baht in Total Investment
BANGKOK, April 5, 2021 /PRNewswire/ -- The Thailand Board of Investment (BOI) said today it has recently approved new projects in the field of advanced biotechnology, worth a combined2.4 billion baht (around USD78 million) in investment, reflecting the increased interest of local and foreign inve...
Sophie's Bionutrients Unveils The World's First Plant-Based Burger Patty Made From Microalgae
SINGAPORE, April 1, 2021 /PRNewswire/ -- Sophie's Bionutrients, a
next-generation sustainable urban food production technology company, has
pushed the frontiers of plant-based food by developing burger patties made from
microalgae.
VISEN's TransCon Human Growth Hormone Phase-III Clinical Trial in China Completes Target Patient Recruitment Number as Scheduled
SHANGHAI, March 31, 2021 /PRNewswire/ -- VISEN Pharmaceuticals, a biotech company committed to the treatment of endocrine-related diseases, announced on March 30 that it had successfully completed its recruiting target of 150 subjects for the Phase-III registration clinical trial inChina to evalu...
Five New 'Key Opinion Leaders' Appointed to Blinkcns Scientific Advisory Board
Dr. Angelo Antonini, Dr. Matteo Bologna, Roy Jones, Dr. Fidias Leon-Sarmiento, and Dr.Fabrizio Stocchi, will help guide company as they advance central nervous system research CHARLESTON, South Carolina, March 31, 2021 /PRNewswire/ -- Blinkcns, Inc, a biotechnology company pioneering blink refle...
BIOVAXYS files FDA pre-IND meeting request and briefing package for COVID-T
VANCOUVER, BC, March 31, 2021 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA:5LB) (OTC:LMNGF) ("BioVaxys"), the world leader in haptenized antigen vaccines for antiviral and cancer applications, announced today that it is has filed a pre-IND (Investigational New Drug) meeting request ...
Evive Biotech Submits Biologics License Application to US FDA for Ryzneuta(TM)
* BLA submission to the U.S. FDA follows successful conclusion of Evive's Global Phase III Clinical Trial for chemotherapy-induced neutropenia in breast cancer patients. * Evive is the first Chinese biologics company to advance a novel biologic product from pre-clinical studies to a complet...
Week's Top Stories
Most Reposted
Amorepacific Showcases Innovative Technologies at CES 2026
[Picked up by 292 media titles]
2026-01-05 12:14Novo Nordisk International Operations Commits to Veeva Vault CRM
[Picked up by 290 media titles]
2026-01-08 09:05Courtyard by Marriott Phuket, Patong Beach Resort: The Ultimate Family-Friendly Destination in 2026
[Picked up by 284 media titles]
2026-01-08 12:01Radisson Hotel Group Revives Bangkok Icon with Opening of Radisson Hotel Chateau de Bangkok
[Picked up by 283 media titles]
2026-01-06 10:45Panduit Announces Key Executive Appointments to Support Strategic Growth and Innovation
[Picked up by 282 media titles]
2026-01-07 10:00