Infectious Disease Control
Asia embraces the dawn
HONG KONG, Dec. 10, 2022 /PRNewswire/ -- A photo exhibition that captures the changes in Asian people's daily lives under the COVID-19 pandemic and encourages people to move forward opened inHong Kong on Friday. Co-organized by China Daily and the Asia News Network, the Changing Asia – New Norma...
Australian Scientists Honoured with the Grand Hamdan Award
DUBAI, UAE, Nov. 25, 2022 /PRNewswire/ -- The Award ceremony of the Sheikh Hamdan bin Rashid Al Maktoum Award for Medical Sciences, hosted in Dubai, took place on 23rd of November the Museum of the Future. The Award celebrated its 12th term (2021-2022) and 22nd year, in the presence of SheikhRas...
Ascletis Announces IND Approval of Oral 3CLpro Inhibitor ASC11 for COVID-19 by U.S. FDA
--The Phase I clinical trial will consist of 3 cohorts in healthy subjects, including single- and multiple-dose escalation studies and food effect study. The objective of Phase I trial is to find a right dose to move into the pivotal Phase II/III in COVID-19 patients --In antiviral cellular assa...
APR Applied Pharma Research (a Subsidiary of Relief Therapeutics) is a Finalist in the 2022 Rare Disease International Film Festival
GENEVA, Nov. 21, 2022 /PRNewswire/ -- RELIEF THERAPEUTICS Holding SA (SIX: RLF) (OTCQB: RLFTF) (RLFTD) ("Relief"), a Swiss, commercial-stage biopharmaceutical company identifying, developing and commercializing novel, patent protected products in selected specialty, rare and ultra-rare disease ar...
Manulife Hong Kong expands partnership with Christian Family Service Centre to support long COVID recovery in underserved communities
New program to provide comprehensive healthcare services to empower sustained health and better wellbeing HONG KONG, Nov. 17, 2022 /PRNewswire/ -- Manulife Hong Kong today announced the launch of "Manulife Health Voucher Program – COVID Recovery" (hereafter referred to as the "Program") in partn...
BioVaxys Sarbecovirus Vaccine Interim Data Excellent Emerging Safety & Tolerability Profile with BVX-1021
VANCOUVER, BC, Nov. 16, 2022 /PRNewswire/ -- BioVaxys Technology Corp. (CSE: BIOV) (FRA: 5LB) (OTCQB: BVAXF) ("BioVaxys" or "Company") is pleased to announce that interim results from its ongoing preclinical of BVX-1021, the Company's vaccine for SARS-CoV-1 ("SARS1") which is being evaluated in a...
Tianlong Showcases Innovative Smart PCR Lab Solution at Medica 2022
XI'AN, CHINA, Nov. 15, 2022 /PRNewswire/ -- From November 14 to 17, Tianlong's newly-launched smart PCR lab solution will showcase at Medica 2022 in Dusseldorf, Germany. The innovative smart PCR lab solution from Tianlong can maximize resources by making the best use of people, space, and equipmen...
Novavax Phase 3 COVID-19 Omicron Trial Supports the Continued and Future Use of Novavax Prototype Vaccine as a Booster
* The Novavax BA.1 vaccine candidate met its primary strain-change endpoint allowing for development of variant vaccines, if necessary * Novavax' prototype vaccine induced broad immune response against original Wuhan, BA.1, and BA.5 strains * The trial showed no benefit for a bivalent vaccin...
Ascletis Announces U.S. IND Filing of Oral 3CLpro Inhibitor ASC11 for COVID-19
-- ASC11 is an in-house discovered oral small molecule drug candidate, targeting 3-chymotrypsin like protease (3CLpro) -- In antiviral cellular assays with infectious SARS-CoV-2, ASC11 demonstrated higher potency against SARS-CoV-2 than other 3CLpro inhibitors including Nirmatrelvir, S-217622, P...
GenScript Supported Nasal Spray Development for COVID-19 Protection with GMP-grade Antibodies and Subsequently Planned for Long-Term Collaboration with Biogenexis
SINGAPORE, Nov. 1, 2022 /PRNewswire/ -- GenScript Biotech Corporation ("GenScript", Stock Code: 1548.HK), a leading global biotechnology company supportedChulalongkorn University, a research-intensive university in Thailand in developing human anti-SARS-CoV-2 antibodies with antibody production a...
U.S. FDA Grants Emergency Use Authorization for Novavax COVID-19 Vaccine, Adjuvanted as a Booster for Adults
GAITHERSBURG, Md., Oct. 22, 2022 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX), a biotechnology company dedicated to developing and commercializing next-generation vaccines for serious infectious diseases, today announced that the Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) has received eme...
Brii Biosciences Presents Promising Clinical Data to Support Ongoing Development of Novel HIV Therapeutic Candidates at IDWeek 2022
* Findings from two Phase 1 studies show both BRII-732 and BRII-778 are safe and well-tolerated * Results indicate potential for oral once-weekly, long-acting combination therapy for HIV infection, supporting continued clinical development * Additional data presented by Brii's licensing part...
The Grand Hamdan Award goes to two scientists in Australia
DUBAI, UAE, Oct. 19, 2022 /PRNewswire/ -- According to an official announcement
made recently inDubai, Prof. Ian Hector Frazer and the Late Dr. Jian Zhou are
the winners of the Grand Hamdan Award for the topic of – Infectious Diseases in
the Award's 12th term (2021-2022).
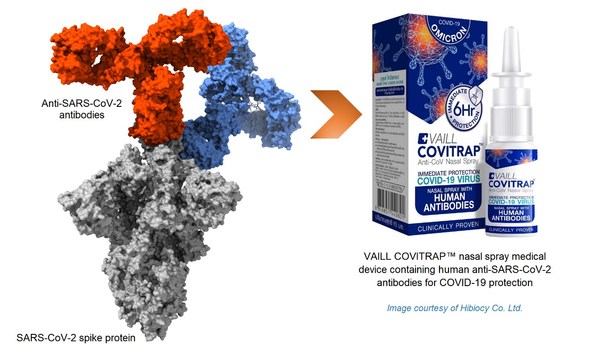
GenScript ProBio Enters Strategic Collaboration with Hibiocy Co. Ltd, the affiliate of Rojukiss International Public Company Limited (KISS) - the leading Thai-based Beauty & Health company, for the development and manufacturing of COVITRAP™ and future new products.
SINGAPORE, Oct. 17, 2022 /PRNewswire/ -- GenScript ProBio (a global one-stop CDMO from GenScript Group) today announced a strategic collaboration with Hibiocy Co. Ltd., a private corporation based inBangkok, Thailand, to accelerate a respiratory-care medical device, COVITRAP™ which contains human...
World Health Summit kicks off with German Chancellor Scholz, numerous Government Officials, WHO Director-General Tedros
Global health in focus BERLIN, Oct. 17, 2022 /PRNewswire/ -- The World Health Summit 2022, the world's leading meeting for global health, began on Sunday morning inBerlin. For the first time it is co-hosted by the World Health Organization (WHO). Among the central topics are climate change and h...
With New Commitment, Gates Foundation Joins Call to Help End Polio
BERLIN, Oct. 17, 2022 /PRNewswire/ -- Today at the World Health Summit, the Bill & Melinda Gates Foundation announced it will commit$1.2 billion to support efforts to end all forms of polio globally. The announcement was made ahead of a key pledging moment that will be co-hosted by Germany and the...
Gilead and the IAS start second year of partnership to honor champions of stigma-free HIV care services in Asia and Latin America
SINGAPORE, Oct. 13, 2022 /PRNewswire/ -- Gilead Sciences, Inc. and IAS – the International AIDS Society – have renewed their Me and My Healthcare Provider partnership to expand the campaign inBrazil, Hong Kong, Mexico, South Korea and Taiwan. The collaborative partnership, now entering its second...
Novavax Prototype COVID-19 Vaccine Data Support Homologous and Heterologous Boosting and Suggest Benefit Against Variants
* Homologous boosting with the prototype Novavax COVID-19 vaccine induced robust antibody titers for Omicron BA.1, BA.2, and BA.5 * Study 307 (Lot Consistency) achieved its primary endpoint, showing that three vaccine lots induced a comparable immune response thereby demonstrating the consist...
Brii Biosciences Announces Top-line Results from Phase 1 Study of BRII-296, A Long-Acting Therapy in Development for Postpartum Depression
* Data demonstrated that a single administration of BRII-296 at 600 mg delivered a favorable pharmacokinetic profile and is safe and well-tolerated in healthy subjects * Findings suggest BRII-296 has potential to provide a safe and effective, one-time injectable treatment option to people wit...
Gates Foundation Announces $1.27 Billion in Health and Development Commitments to Advance Progress Toward the Global Goals
First in-person Goalkeepers event since 2019 convenes global changemakers to highlight the urgency of achieving a more equitable world by 2030 NEW YORK, Sept. 22, 2022 /PRNewswire/ -- During United Nations General Assembly week, the Bill & Melinda Gates Foundation—alongside governments, philanth...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 327 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 302 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09