Medical/Pharmaceuticals
Explore "Intensive Care Airway Clearance Technology" with BMC at ESICM 2024
BARCELONA, Spain, Oct. 11, 2024 /PRNewswire/ -- Barcelona, renowned for its rich cultural heritage and artistic vibrancy, is also a key player in the global medical research and innovation landscape. Home to several prestigious medical schools, hospitals, and cutting-edge research institutions, t...
HMI Medical Announces Partnership with Advanced Urology Associates to Expand its Specialist Service Offerings
* Advanced Urology Associates, the largest private urology specialist group in Singapore, joins HMI Medical through a majority stake acquisition to meet growing regional demand for comprehensive urology care * With Advanced Urology Associates, HMI Medical further enhances their one-stop health...
Delegation of International Communication Experts and Foreign Media Outlets Visits Emerging Industry Enterprises in Zhongshan, Guangdong Province
BEIJING, Oct. 11, 2024 /PRNewswire/ -- On October 9, 2024, the delegation of international communication experts and foreign media outlets, themed "The World Comes to Zhongshan" and organized by the CICG Academy of Translation and Interpretation, visited several representative enterprises in Zhon...
KeyBioscience Announces Extension of Strategic Collaboration with Lilly
Companies to extend collaboration on DACRAs with an additional collaboration molecule and investigation of the DACRA platform within multiple indications LUGANO, Switzerland, Oct. 10, 2024 /PRNewswire/ -- KeyBioscience and Eli Lilly and Company have agreed to extend their collaboration on the dev...
AIA Club Alta celebrates first anniversary Introducing brand-new infinite health programmes
Expanding wealth management services to include family office setup consultation and referral Launching special anniversary event: "AIA Alta Presents the World Circus" HONG KONG, Oct. 10, 2024 /PRNewswire/ -- AIA Club Alta provides one-stop wealth management, health and wellness service privilege...
Newbase Inc. Unveils AI-Based VR Medical Education Platform 'Medicrew'
Introducing the AI-Powered Meta-Hospital Education Platform 'Medicrew' at the 2024 Hong Kong Autumn Electronics Fair SEOUL, South Korea, Oct. 10, 2024 /PRNewswire/ -- Medical edtech company Newbase will be showcasing its innovative meta-hospital education platform 'Medicrew' at the 2024 Hong Kon...
ICCBBA Announces 2024 Enterprise Grant Awardees: Advancing Global Blood Safety
REDLANDS, Calif., Oct. 10, 2024 /PRNewswire/ -- ICCBBA is proud to announce the recipients of its2024 Enterprise Grant Awards, recognizing three outstanding organizations that are advancing patient safety, blood accessibility, and quality systems through innovative projects inAfrica. These grants...
USANA's First Become A USANA Coach Program: A Major Success and a Step Toward Healthier Communities
KUALA LUMPUR, Malaysia, Oct. 10, 2024 /PRNewswire/ -- USANA is thrilled to announce the resounding success of our first Become A USANA Coach Program, held at The Vertical, Connexion Conference & Event Centre, Grand Summit Ballroom (Level M1), Bangsar South. This event attracted over 970 passionat...
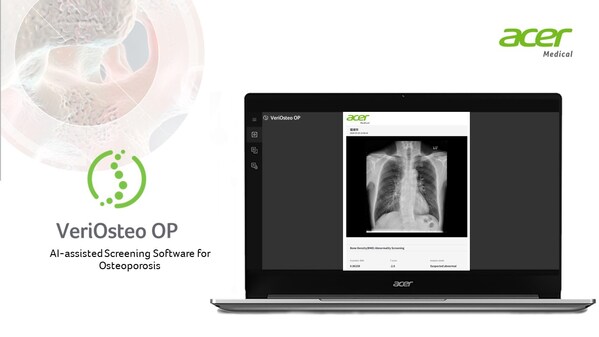
Acer Medical: VeriOsteo OP Obtains Medical Device Approval from Indonesia's Ministry of Health
TAIPEI, Oct. 10, 2024 /PRNewswire/ -- Acer Medical announced that its AI-assisted bone mineral density abnormalities screening software, VeriOsteo OP, has obtained a medical device certificate fromIndonesia's Ministry of Health. This marks the second product to receive registration approval, fol...
Daewoong Pharmaceutical Unveils 'Innovative Drug Delivery Technologies' in Milan--From World's First Microneedle Therapy to Once-a-Month Obesity Treatment
MILAN, Oct. 10, 2024 /PRNewswire/ -- Daewoong Pharmaceutical (Co-CEOs Seongsoo Park and Chang-jae Lee) announced on the 9th that they will present their cutting-edge drug delivery technologies, including the world's first microneedle-based drugs, at 'CPHI Worldwide 2024'—the world's largest phar...
Centivax Selects Global CDMO BioCina to Initiate cGMP Manufacturing of Revolutionary Universal Influenza Vaccine
ADELAIDE, South Australia, Oct. 9, 2024 /PRNewswire/ -- BioCina Pty Ltd., a global end-to-end biologics Contract Development and Manufacturing Organization (CDMO), announced a new partnership with Centivax, Inc., for a project involving cell line development, cell banking and plasmid DNA manufact...
Inaugural Health and Wellbeing Forum Discussed about Co-creating Healthier Hong Kong for all
This event brought together over 60 representatives from patient organizations, academia, and healthcare community to explore the possibilities of achieving maximum health equity inHong Kong HONG KONG, Oct. 9, 2024 /PRNewswire/ -- Pfizer Hong Kong held its inaugural "Health & Well-being Forum" t...
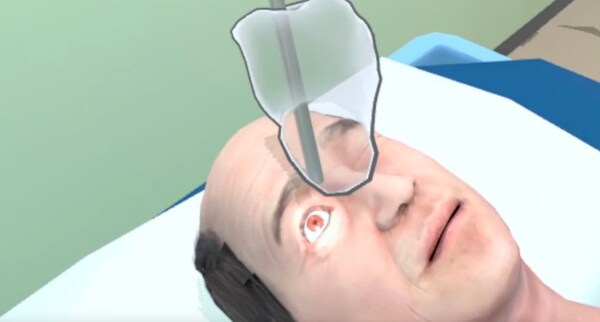
ZEISS expands ophthalmic offerings to improve patient care with new digital AI tools and revolutionary surgical solutions
Showcasing at AAO 2024: * ZEISS VisioGen offers a cutting-edge, AI-driven solution designed to enhance refractive patient communication and streamline clinic operations. * ZEISS Milestone: Celebrating more than 10 million eyes treated with lenticule extraction solutions utilizing ZEISS SMIL...
ShiraTronics Raises $66 Million in Oversubscribed Series B Financing to Advance Pioneering Treatment for Chronic Migraine
Implantable Neuromodulation System Shows Early Promise in Improving Patient Quality of Life by Reducing Monthly Headache Days and Severity of Migraine Attacks MINNEAPOLIS, Oct. 9, 2024 /PRNewswire/ -- ShiraTronics, a clinical-stage medical device company developing neurostimulation therapies fo...
International Collaboration Addresses Rising Cancer Rates in South America
National Comprehensive Cancer Network and Latin American Cooperative Oncology Group to publish cancer treatment guidelines tailored forBrazil. PLYMOUTH MEETING, Pa., Oct. 9, 2024 /PRNewswire/ -- The National Comprehensive Cancer Network® (NCCN®)—an alliance of leading cancer centers in the Unite...
New INTREPID Alliance Antiviral Landscape Report Highlights Urgent Need to Bolster R&D Pipeline in Support of Global Pandemic Preparedness Efforts
Current mpox outbreak underscores gaps in access to medical countermeasures to help mitigate the severity of future outbreaks CAMBRIDGE, Mass., Oct. 9, 2024 /PRNewswire/ -- Today, the INTREPID Alliance, a consortium of innovative biopharmaceutical companies dedicated to accelerating antiviral tr...
The CDE has Granted Breakthrough Therapy Designation to LBL-024, a Bispecific Antibody that Targets PD-L1 and 4-1BB, Developed by Leads Biolabs, for the Treatment of Extrapulmonary Neuroendocrine Carcinoma.
NANJING, China, Oct. 9, 2024 /PRNewswire/ -- Nanjing Leads Biolabs Co., Ltd. ("Leads Biolabs") announced that Center for Drug Evaluation (CDE) of National Medical Products Administration (NMPA) has granted Breakthrough Therapy Designation to LBL-024, an anti-PD-L1/4-1BB bispecific antibody indepe...
AWAK Transforms into Vivance, Unveiling a New Brand Identity
SINGAPORE, Oct. 9, 2024 /PRNewswire/ -- AWAK Technologies, a pioneer in innovative kidney care is excited to announce its rebranding toVivance. The name, derived from 'viva' and 'advance', embodies vitality and progress - hallmarks of the company's commitment to revolutionizing kidney health. AW...
Seegene and Werfen Finalize Partnership Agreement on Technology-Sharing Initiative
* Werfen, a worldwide leader in specialized diagnostics, becomes the first European company to finalize the partnership agreement to join Seegene's technology-sharing initiative * The two companies will co-establish a NewCo in Spain to serve as a strategic hub inEurope, accelerating global ex...
LakeShore Biopharma Provides Fiscal Half Year 2025 Financial Guidance and Reaffirms Fiscal Year 2025 Financial Guidance
GAITHERSBURG, Md., Oct. 8, 2024 /PRNewswire/ -- LakeShore Biopharma Co., Ltd (Nasdaq: LSB) ("LakeShore Biopharma" or the "Company"), a global biopharmaceutical company dedicated to discovering, developing, manufacturing, and delivering new generations of vaccines and therapeutic biologics for in...
Week's Top Stories
Most Reposted
Marina Bay precinct partners UOB, Marina Bay Sands and Singapore Tourism Board, together with Disney Cruise Line, to illuminate Singapore's skyline with a fireworks sky show
[Picked up by 328 media titles]
2026-02-19 14:30Never Miss a Message: Agoda's Customer Support Now Travels With You
[Picked up by 326 media titles]
2026-02-24 12:00NextFin Asia: A New Dedicated Fund for the Catapult: Inclusion SE Asia Program to Further Scale Inclusive Finance Fintechs in ASEAN
[Picked up by 311 media titles]
2026-02-23 08:00Klook and Osaka Convention & Tourism Bureau sign MoU to advance inbound tourism and foster socio-economic development throughout Osaka Prefecture
[Picked up by 301 media titles]
2026-02-24 16:13Vitafoods Asia 2026 Expands by 30%: A Bigger, More Dynamic Trade Event with Exciting New Features & Increased International Participation
[Picked up by 288 media titles]
2026-02-23 10:09