Medical/Pharmaceuticals
Daewoong Pharmaceutical's Envlo to enter the global market in full swing with filing for product license in three ASEAN countries
* Submitted an NDA to Indonesia, Philippines and Thailand * Signed the export contract in Brazil and Mexico in last February * Aiming to enter 50 countries by 2030 SEOUL, South Korea, March 22, 2023 /PRNewswire/ -- Daewoong Pharmaceutical began the advance into the global market in full swin...
Turacoz's Research & Trainings Empowering Healthcare Professionals with Enhanced Publication Skills
UTRECHT, Netherlands and NEW DELHI, India, March 22, 2023 /PRNewswire/ -- Healthcare professionals' busy schedules and easy access to social media have reduced their attention spans, making it difficult to read lengthy research papers. Fortunately, enhanced publications summarize research in vid...
Dignitana enters Hong Kong market with leading distributor Science International Corporation
LUND, Sweden, March 22, 2023 /PRNewswire/ -- Scalp cooling innovator Dignitana AB has signed Science International Corporation, a leading distributor for medical devices, to launch The DigniCap Scalp Cooling System inHong Kong and Macao. Science International now becomes the exclusive provider of...
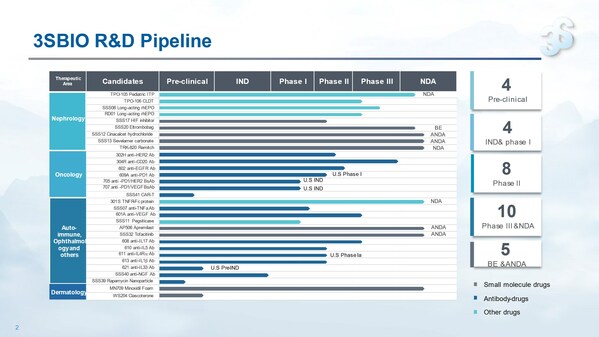
3SBio announces 2022 annual results, with revenue rising 7.5% year on year and normalized net profit attributable to parents jumping 25.2%
HONG KONG, March 22, 2023 /PRNewswire/ -- Chinese leading biopharmaceutical company 3SBio (01530.HK) today announced its 2022 annual results. In 2022, 3SBio's core biopharmaceutical products posted robust growth, hair health business achieved blistering performance, and contract development and ...
APACMed Names EVERSANA Knowledge Partner to Shape Digital Health Reimbursement Framework Across Region
SINGAPORE, March 22, 2023 /PRNewswire/ -- Asia Pacific Medical Technology Association (APACMed), the region's leading organization for medical technology manufacturers, has named EVERSANA®, a pioneer of next-generation commercial services to the global life sciences industry and a market leader ...
DEBIOPHARM ANNOUNCES LAUNCH OF THE PHASE 1/2 GaLuCi™ STUDY FOR ITS CA IX-TARGETED RADIOPHARMACEUTICAL PROGRAM
* Debiopharm is developing personalized radiotherapy through a theranostic approach, combiningdiagnostic imaging (Debio 0328 a gallium-labelled imaging tool) and therapeutic components (Debio 0228, a lutetium-labelled radioligand), thus allowing the pre-identification and treatment of patients ...
See-Mode Technologies Secures Regulatory Approvals for its AI-powered Software that Automatically Analyses and Reports Breast & Thyroid Ultrasound Scans in Australia and New Zealand
MELBOURNE, Australia, March 22, 2023 /PRNewswire/ -- See-Mode Technologies, a leading provider of AI-powered medical imaging solutions, today announced that it has received regulatory approvals for its AI-powered breast and thyroid ultrasound solution inAustralia and New Zealand. These approvals ...
Two-Component Recombinant COVID-19 Vaccine ReCOV Granted With Emergency Use Authorization In Mongolia
TAIZHOU, China, March 21, 2023 /PRNewswire/ -- Jiangsu Recbio Technology Co., Ltd. (the "Company", together with its subsidiaries, the "Group") is pleased to announce that in accordance with the Mongolian law on the prevention of novel coronavirus (SARS-CoV-2) outbreak, the Company was granted an...
BLA Accepted in China for Boan Biotech's Denosumab Injection (BA1102) for the Oncology Indications
YANTAI, China, March 21, 2023 /PRNewswire/ -- Luye Pharma Group today announced that the Biologics License Application (BLA) for BA1102 (Denosumab Injection), a biosimilar for the oncology indications developed by its subsidiary Boan Biotech, has been accepted inChina by the Center for Drug Evalu...
Health Food Symmetry has formed a strategic alignment with Sunrise Health & Wellness launching new products at the 2023 Australian Pharmacy Professional Conference & Trade Exhibition (Gold Coast, March 23 - 26)
Health and Wellness Market Disruption – New Partnership SYDNEY, March 21, 2023 /PRNewswire/ -- Health Food Symmetry (HFS) and Sunrise Health and Wellness (Sunrise) are excited to announce the strategic alignment that will see the two organisations join forces to help accelerate the brand develop...
Innovent Announces Clinical Data of Multiple Trials Will be Presented at the AACR Annual Meeting 2023
ROCKVILLE, Md. and SUZHOU, China, March 21, 2023 /PRNewswire/ -- Innovent Biologics, Inc. ("Innovent") (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of oncology, autoimmune, metabolic, ophthalmology ...
Sirnaomics to Present Latest Developments in Polypeptide Nanoparticle Platform at the 4th Annual RNAi-Based Therapeutics Summit
HONG KONG and GERMANTOWN, Md. and SUZHOU BIOBAY, China, March 20, 2023 /PRNewswire/ -- Sirnaomics Ltd. (the "Company", Stock Code: 2257.HK, and together with its subsidiaries, the"Group" or "Sirnaomics"), a leading biopharmaceutical company in the discovery and development of advanced RNAi thera...
Transcenta Holding Limited (6628.HK) to Hold 2022 Annual Results Release Conference Call
SUZHOU, China, March 20, 2023 /PRNewswire/ -- The management team of Transcenta Holding Limited (06628.HK) will attend the 2022 annual results release conference call onMarch 31, 2023. Transcenta is a clinical stage biopharmaceutical company with fully integrated capabilities in the discovery, r...
Building Back: Helping Young People Go from Recovery to Discovery
Outward Bound International releases timely and relevant report about the role of outdoor learning in addressing youth mental health and helping young people build back post pandemic. NEW YORK, March 20, 2023 /PRNewswire/ -- Outward Bound International, the coordinating organization for Outward ...
Guo Guangchang Stresses Healthy Corporate Development and Expresses Full Confidence in the Future
HONG KONG, March 20, 2023 /PRNewswire/ -- On 17 and 18 March, Guo Guangchang, Chairman of Fosun International, attended the 23rd Annual Conference of Yabuli China Entrepreneurs Forum 2023, and the 6th Shanghai Forum Themed Zhejiang Entrepreneurs in the World & the 11th Member Representative Confe...
ETANA HAS SECURED FINANCING FROM DEG, EAST VENTURES AND OTHER GLOBAL INVESTORS
JAKARTA, Indonesia, March 20, 2023 /PRNewswire/ -- PT Etana Biotechnologies Indonesia (Etana), an Indonesian biopharmaceutical company, has secured a new round of investment led by DEG followed by Yunfeng Capital, HighLight Capital and East Ventures. This round of financing will be used for fu...
Antengene Announces Five Upcoming Presentations at the 2023 American Association for Cancer Research Annual Meeting
– Five posters will showcase progress with multiple preclinical and clinical programs, including the clinical results ofATG-008 (mTORC1/2 inhibitor) and preclinical data ofATG-031 (anti-CD24 monoclonal antibody), ATG-037 (small molecule CD73 inhibitor), ATG-017 (ERK1/2 inhibitor), and ATG-034 (L...
Agilis Robotics has kickstarted its first trial in mainland China, taking a great leap forward in robotized endoscopic surgery in the country
HONG KONG, March 20, 2023 /PRNewswire/ -- Agilis Robotics is a company that is revolutionizing the world of endoscopic surgery. The company has developed the world's smallest flexible and dexterous endoscopic robot system, which is a breakthrough in the field of robotics surgery. The Agilis Robo...
Innovating for better sleep
BEIJING, March 20, 2023 /PRNewswire/ -- Sleep is essential for health. Like eating well and exercising, sleep is a behavior that is essential to a person's physical, mental, and social well-being. However, sleep is not yet universally recognized as an essential behavior for physical health.1 BMC...
320 World Oral Health Day - Lifting the "mask mandate", maintaining good oral hygiene and flashing a healthy and confident smile.
HONG KONG, March 20, 2023 /PRNewswire/ -- According to a report released by the World Health Organization (WHO) in 2022, oral diseases are the most common non-communicable diseases in humans and affect 3.5 billion people worldwide. Approximately 2 billion people have untreated caries in permanent...
Week's Top Stories
Most Reposted
COCONEXT 2024 - FIRST EVER INTERNATIONAL COCONUT CONFERENCE HOLD IN VIETNAM
[Picked up by 301 media titles]
2024-12-31 14:18Dahua Technology Obtains ISO 37301 Compliance Management System Certification
[Picked up by 283 media titles]
2024-12-30 15:33Interest-Driven Consumption Sparks ¥ 500B ACG Goods Market, MINISO Rides the Wave
[Picked up by 282 media titles]
2025-01-03 19:33Russell Reynolds Associates Appoints Euan Kenworthy as Singapore Country Manager
[Picked up by 278 media titles]
2025-01-02 08:16CKGSB Professor Mei Jianping Launches Global Indices Tracking Impressionist, Contemporary, and Chinese Art Markets
[Picked up by 260 media titles]
2024-12-31 22:30